Early treatment with fluvoxamine, bromhexine, cyproheptadine, and niclosamide to prevent clinical deterioration in patients with symptomatic COVID-19: a randomized clinical trial
- PMID: 38516100
- PMCID: PMC10955208
- DOI: 10.1016/j.eclinm.2024.102517
Early treatment with fluvoxamine, bromhexine, cyproheptadine, and niclosamide to prevent clinical deterioration in patients with symptomatic COVID-19: a randomized clinical trial
Abstract
Background: Repurposed drugs with host-directed antiviral and immunomodulatory properties have shown promise in the treatment of COVID-19, but few trials have studied combinations of these agents. The aim of this trial was to assess the effectiveness of affordable, widely available, repurposed drugs used in combination for treatment of COVID-19, which may be particularly relevant to low-resource countries.
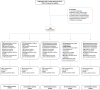
Methods: We conducted an open-label, randomized, outpatient, controlled trial in Thailand from October 1, 2021, to June 21, 2022, to assess whether early treatment within 48-h of symptoms onset with combinations of fluvoxamine, bromhexine, cyproheptadine, and niclosamide, given to adults with confirmed mild SARS-CoV-2 infection, can prevent 28-day clinical deterioration compared to standard care. Participants were randomly assigned to receive treatment with fluvoxamine alone, fluvoxamine + bromhexine, fluvoxamine + cyproheptadine, niclosamide + bromhexine, or standard care. The primary outcome measured was clinical deterioration within 9, 14, or 28 days using a 6-point ordinal scale. This trial is registered with ClinicalTrials.gov (NCT05087381).
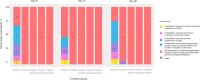
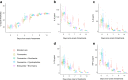
Findings: Among 1900 recruited, a total of 995 participants completed the trial. No participants had clinical deterioration by day 9, 14, or 28 days among those treated with fluvoxamine plus bromhexine (0%), fluvoxamine plus cyproheptadine (0%), or niclosamide plus bromhexine (0%). Nine participants (5.6%) in the fluvoxamine arm had clinical deterioration by day 28, requiring low-flow oxygen. In contrast, most standard care arm participants had clinical deterioration by 9, 14, and 28 days. By day 9, 32.7% (110) of patients in the standard care arm had been hospitalized without requiring supplemental oxygen but needing ongoing medical care. By day 28, this percentage increased to 37.5% (21). Additionally, 20.8% (70) of patients in the standard care arm required low-flow oxygen by day 9, and 12.5% (16) needed non-invasive or mechanical ventilation by day 28. All treated groups significantly differed from the standard care group by days 9, 14, and 28 (p < 0.0001). Also, by day 28, the three 2-drug treatments were significantly better than the fluvoxamine arm (p < 0.0001). No deaths occurred in any study group. Compared to standard care, participants treated with the combination agents had significantly decreased viral loads as early as day 3 of treatment (p < 0.0001), decreased levels of serum cytokines interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β) as early as day 5 of treatment, and interleukin-8 (IL-8) by day 7 of treatment (p < 0.0001) and lower incidence of post-acute sequelae of COVID-19 (PASC) symptoms (p < 0.0001). 23 serious adverse events occurred in the standard care arm, while only 1 serious adverse event was reported in the fluvoxamine arm, and zero serious adverse events occurred in the other arms.
Interpretation: Early treatment with these combinations among outpatients diagnosed with COVID-19 was associated with lower likelihood of clinical deterioration, and with significant and rapid reduction in the viral load and serum cytokines, and with lower burden of PASC symptoms. When started very soon after symptom onset, these repurposed drugs have high potential to prevent clinical deterioration and death in vaccinated and unvaccinated COVID-19 patients.
Funding: Ped Thai Su Phai (Thai Ducks Fighting Danger) social giver group.
Keywords: Bromhexine; COVID-19 treatment; Cyproheptadine; Early treatment; Fluvoxamine; Niclosamide.
© 2024 The Author(s).
Conflict of interest statement
Dr. Reiersen is listed as an inventor on a patent application related to methods of treating COVID-19 (including Sigma1 agonists and specifically fluvoxamine), which was filed by Washington University in St. Louis. No other author declares any potential conflict of interest or competing financial or non-financial interest in relation to the manuscript.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

