Prescribing and deprescribing guidance for benzodiazepine and benzodiazepine receptor agonist use in adults with depression, anxiety, and insomnia: an international scoping review
- PMID: 38516102
- PMCID: PMC10955669
- DOI: 10.1016/j.eclinm.2024.102507
Prescribing and deprescribing guidance for benzodiazepine and benzodiazepine receptor agonist use in adults with depression, anxiety, and insomnia: an international scoping review
Abstract
Background: Clinical practice guidelines and guidance documents routinely offer prescribing clinicians' recommendations and instruction on the use of psychotropic drugs for mental illness. We sought to characterise parameters relevant to prescribing and deprescribing of benzodiazepine (BZD) and benzodiazepine receptor agonist (BZRA), in clinical practice guidelines and guidance documents internationally, for adult patients with unipolar depression, anxiety disorders and insomnia to understand similarities and discrepancies between evidence-based expert opinion.
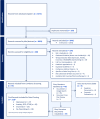
Methods: A Scoping Review was conducted to characterize documents that offered evidence-based and/or consensus pharmacologic guidance on the management of unipolar depression, anxiety disorders, obsessive-compulsive disorders, post-traumatic stress disorders and insomnia. A systematic search was conducted of PubMed, SCOPUS, PsycINFO and CINAHL from inception to October 13, 2023 and supplemented by a gray literature search. Documents were screened in Covidence for eligibility. Subsequent data-charting on eligible documents collected information on aspects of both prescribing and deprescribing.
Findings: 113 documents offering guidance on BZD/BZRA use were data-charted. Overall, documents gathered were from Asia (n = 11), Europe (n = 34), North America (n = 37), Oceania (n = 7), and South America (n = 4) with the remainder being "International" (n = 20) and not representative to any particular region or country. By condition the documents reviewed covered unipolar depressive disorders (n = 28), anxiety disorders, obsessive-compulsive disorder and post-traumatic stress disorder (n = 42) and Insomnia (n = 25). Few documents (n = 18) were sufficiently specific and complete to consider as de-prescribing focused documents.
Interpretation: Documents were in concordance in terms of BZD and BZRA not being used routinely as first-line pharmacologic agents. When used, it is advisable to restrict their duration to "short-term" use with the most commonly recommended duration being less than four weeks. Documents were less consistent in terms of prescriptive recommendations for specific drug, dosing and administration pattern (i.e regular or 'as needed') selection for each condition. Deprescribing documents were unanimously in favor of gradual dose reduction and patient shared decision-making. However, approaches towards dose-tapering differed substantially. Finally, there were inconsistencies and/or insufficiency of detail, among deprescribing documents, in terms of switching to a long-acting BZD, use of adjunctive pharmacotherapies and micro-tapering.
Funding: The authors received no funding for this work.
Keywords: Anxiety; Benzodiazepines; Clinical practice guidelines; Deprescribing; Depression; Insomnia.
© 2024 The Author(s).
Conflict of interest statement
Brandt, Bressi, Cadogan, Witt-Doerring (M & J) and Wright have all served as either advisors or directors to the Alliance for Benzodiazepine Best Practices; a not-for-profit organization with the mission to inform evidence-based improvements in the use of benzodiazepines and Z-drugs. Wright has received personal payment (unrelated to this project) for his past service as medical director of the Alliance for Benzodiazepine Best Practices.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

