Increasing the Dissolution Rate of Polystyrene Waste in Solvent-Based Recycling
- PMID: 38516401
- PMCID: PMC10952012
- DOI: 10.1021/acssuschemeng.3c08154
Increasing the Dissolution Rate of Polystyrene Waste in Solvent-Based Recycling
Abstract
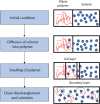
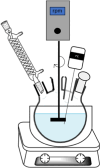
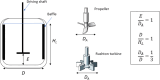
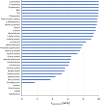
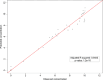
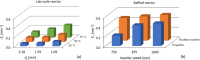
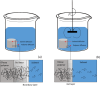
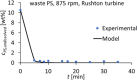
Solvent-based recycling of plastic waste is a promising approach for cleaning polymer chains without breaking them. However, the time required to actually dissolve the polymer in a lab environment can take hours. Different factors play a role in polymer dissolution, including temperature, turbulence, and solvent properties. This work provides insights into bottlenecks and opportunities to increase the dissolution rate of polystyrene in solvents. The paper starts with a broad solvent screening in which the dissolution times are compared. Based on the experimental results, a multiple regression model is constructed, which shows that within several solvent properties, the viscosity of the solvent is the major contributor to the dissolution time, followed by the hydrogen, polar, and dispersion bonding (solubility) parameters. These results also indicate that cyclohexene, 2-pentanone, ethylbenzene, and methyl ethyl ketone are solvents that allow fast dissolution. Next, the dissolution kinetics of polystyrene in cyclohexene in a lab-scale reactor and a baffled reactor are investigated. The effects of temperature, particle size, impeller speed, and impeller type were studied. The results show that increased turbulence in a baffled reactor can decrease the dissolution time from 40 to 7 min compared to a lab-scale reactor, indicating the importance of a proper reactor design. The application of a first-order kinetic model confirms that dissolution in a baffled reactor is at least 5-fold faster than that in a lab-scale reactor. Finally, the dissolution kinetics of a real waste sample reveal that, in optimized conditions, full dissolution occurs after 5 min.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Model analysis on effect of temperature on the solubility of recycling of Polyethylene Terephthalate (PET) plastic.Chemosphere. 2022 Nov;307(Pt 3):136050. doi: 10.1016/j.chemosphere.2022.136050. Epub 2022 Aug 14. Chemosphere. 2022. PMID: 35977561
-
Computational Approach for Rapidly Predicting Temperature-Dependent Polymer Solubilities Using Molecular-Scale Models.ChemSusChem. 2021 Oct 5;14(19):4307-4316. doi: 10.1002/cssc.202101137. Epub 2021 Jul 29. ChemSusChem. 2021. PMID: 34240559
-
Study of the solubility and stability of polystyrene wastes in a dissolution recycling process.Waste Manag. 2009 Jun;29(6):1814-8. doi: 10.1016/j.wasman.2009.01.001. Epub 2009 Feb 12. Waste Manag. 2009. PMID: 19217275
-
Dissolution-Precipitation of Plastic Waste: Current Position and Potential as Green Recycling Technique.ChemSusChem. 2025 Jun 2;18(11):e202500018. doi: 10.1002/cssc.202500018. Epub 2025 Mar 6. ChemSusChem. 2025. PMID: 40047793 Review.
-
State-Of-The-Art Quantification of Polymer Solution Viscosity for Plastic Waste Recycling.ChemSusChem. 2021 Oct 5;14(19):4071-4102. doi: 10.1002/cssc.202100876. Epub 2021 Aug 27. ChemSusChem. 2021. PMID: 34324273 Free PMC article. Review.
Cited by
-
Repurposing Laboratory Plastic into Functional Fibrous Scaffolds via Green Electrospinning for Cell Culture and Tissue Engineering Applications.ACS Biomater Sci Eng. 2025 Jun 9;11(6):3573-3585. doi: 10.1021/acsbiomaterials.5c00146. Epub 2025 Apr 10. ACS Biomater Sci Eng. 2025. PMID: 40207865 Free PMC article.
-
Sustainable Multi-Cycle Physical Recycling of Expanded Polystyrene Waste for Direct Ink Write 3D Printing and Casting: Analysis of Mechanical Properties.Polymers (Basel). 2024 Dec 23;16(24):3609. doi: 10.3390/polym16243609. Polymers (Basel). 2024. PMID: 39771459 Free PMC article.
References
-
- Miller-Chou B. A.; Koenig J. L. A Review of Polymer Dissolution. Prog. Polym. Sci. 2003, 28 (8), 1223–1270. 10.1016/S0079-6700(03)00045-5. - DOI
-
- Papanu J. S.; Soane Soong D. S.; Bell A. T.; Hess D. W. Transport Models for Swelling and Dissolution of Thin Polymer Films. J. Appl. Polym. Sci. 1989, 38 (5), 859–885. 10.1002/app.1989.070380509. - DOI
-
- Peppas N. A.; Wu J. C.; von Meerwall E. D. Mathematical Modeling and Experimental Characterization of Polymer Dissolution. Macromolecules 1994, 27 (20), 5626–5638. 10.1021/ma00098a017. - DOI
-
- Ghasemi M.; Singapati A. Y.; Tsianou M.; Alexandridis P. Dissolution of Semicrystalline Polymer Fibers: Numerical Modeling and Parametric Analysis. AIChE J. 2017, 63 (4), 1368–1383. 10.1002/aic.15615. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
