Engineering Enzymes for Environmental Sustainability
- PMID: 38516574
- PMCID: PMC10952289
- DOI: 10.1002/ange.202309305
Engineering Enzymes for Environmental Sustainability
Abstract
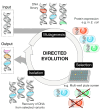
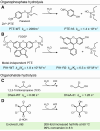
The development and implementation of sustainable catalytic technologies is key to delivering our net-zero targets. Here we review how engineered enzymes, with a focus on those developed using directed evolution, can be deployed to improve the sustainability of numerous processes and help to conserve our environment. Efficient and robust biocatalysts have been engineered to capture carbon dioxide (CO2) and have been embedded into new efficient metabolic CO2 fixation pathways. Enzymes have been refined for bioremediation, enhancing their ability to degrade toxic and harmful pollutants. Biocatalytic recycling is gaining momentum, with engineered cutinases and PETases developed for the depolymerization of the abundant plastic, polyethylene terephthalate (PET). Finally, biocatalytic approaches for accessing petroleum-based feedstocks and chemicals are expanding, using optimized enzymes to convert plant biomass into biofuels or other high value products. Through these examples, we hope to illustrate how enzyme engineering and biocatalysis can contribute to the development of cleaner and more efficient chemical industry.
This review highlights how engineered enzymes have been developed and implemented to help address environmental challenges. Topics include the use of engineered enzymes for improving carbon capture and utilisation, bioremediation, plastic deconstruction, and renewable feedstock generation. Successes, challenges, and opportunities for future enzyme engineering campaigns to improve environmental sustainability are discussed.
Keywords: Biocatalysis; Directed Evolution; Enzyme Engineering; Sustainability.
© 2023 The Authors. Angewandte Chemie published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- “World Population Prospects 2022: Summary of Results.: UN DESA POP”, https://www.un.org/development/desa/pd/content/World-Population-Prospect... 2022.
-
- “2021 TRI National Analysis”, https://www.epa.gov/trinationalanalysis 2021.
-
- “Plastic Waste Makers Index”, https://www.minderoo.org/plastic-waste-makers-index 2023.
-
- “Transforming our world: the 2030 Agenda for Sustainable Development | Department of Economic and Social Affairs,” https://sdgs.un.org/2030agenda, 2015.
Publication types
LinkOut - more resources
Full Text Sources
