Application of tobramycin benzyl ether as an antibiotic adjuvant capable of sensitizing multidrug-resistant Gram-negative bacteria to rifampicin
- PMID: 38516601
- PMCID: PMC10953491
- DOI: 10.1039/d3md00602f
Application of tobramycin benzyl ether as an antibiotic adjuvant capable of sensitizing multidrug-resistant Gram-negative bacteria to rifampicin
Abstract
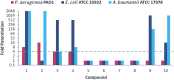
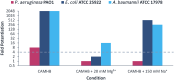
The emergence of aminoglycoside resistance has prompted the development of amphiphilic aminoglycoside derivatives which target bacterial membranes. Tobramycin and nebramine ether derivatives initially designed for this purpose were optimized and screened for their potential application as outer membrane (OM) permeabilizing adjuvants. Structure-activity relationship (SAR) studies revealed that the tobramycin benzyl ether was the most optimal OM permeabilizer, capable of potentiating rifampicin, novobiocin, vancomycin, minocycline, and doxycycline against Gram-negative bacteria. The innovative use of this compound as an adjuvant is highlighted by its ability to sensitize multidrug-resistant (MDR) Gram-negative bacteria to rifampicin and restore the susceptibility of MDR Escherichia coli to minocycline.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Zhang Y. Zhang N. Wang M. Luo M. Peng Y. Li Z. Xu J. Ou M. Kan B. Li X. Lu X. Biosaf. Health. 2023;5:14–20. doi: 10.1016/j.bsheal.2023.01.001. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

