Use of Inhaled Epoprostenol in Patients With COVID-19 Receiving Humidified, High-Flow Nasal Oxygen Is Associated With Progressive Respiratory Failure
- PMID: 38516615
- PMCID: PMC10956404
- DOI: 10.1016/j.chstcc.2023.100019
Use of Inhaled Epoprostenol in Patients With COVID-19 Receiving Humidified, High-Flow Nasal Oxygen Is Associated With Progressive Respiratory Failure
Abstract
Background: The clinical benefit of using inhaled epoprostenol (iEpo) through a humidified high-flow nasal cannula (HHFNC) remains unknown for patients with COVID-19.
Research question: Can iEpo prevent respiratory deterioration for patients with positive SARS-CoV-2 findings receiving HHFNC?
Study design and methods: This multicenter retrospective cohort analysis included patients aged 18 years or older with COVID-19 pneumonia who required HHFNC treatment. Patients who received iEpo were propensity score matched to patients who did not receive iEpo. The primary outcome was time to mechanical ventilation or death without mechanical ventilation and was assessed using Kaplan-Meier curves and Cox proportional hazard ratios. The effects of residual confounding were assessed using a multilevel analysis, and a secondary analysis adjusted for outcome propensity also was performed in a multivariable model that included the entire (unmatched) patient cohort.
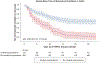
Results: Among 954 patients with positive SARS-CoV-2 findings receiving HHFNC therapy, 133 patients (13.9%) received iEpo. After propensity score matching, the median number of days until the composite outcome was similar between treatment groups (iEpo: 5.0 days [interquartile range, 2.0-10.0 days] vs no-iEpo: 6.5 days [interquartile range, 2.0-11.0 days]; P = .26), but patients who received iEpo were more likely to meet the composite outcome in the propensity score-matched, multilevel, and multivariable unmatched analyses (hazard ratio, 2.08 [95% CI, 1.73-2.50]; OR, 4.72 [95% CI, 3.01-7.41]; and OR, 1.35 [95% CI, 1.23-1.49]; respectively).
Interpretation: In patients with COVID-19 receiving HHFNC therapy, use of iEpo was associated with the need for invasive mechanical ventilation.
Keywords: ARDS; Acute respiratory distress syndrome; COVID-19; SARS-CoV-2; humidified high-flow nasal cannula; inhaled epoprostenol; inhaled pulmonary vasodilators.
Figures

Similar articles
-
Evaluation of Continuous Inhaled Epoprostenol in the Treatment of Acute Respiratory Distress Syndrome, Including Patients With SARS-CoV-2 Infection.Ann Pharmacother. 2022 Oct;56(10):1093-1099. doi: 10.1177/10600280211069182. Epub 2022 Jan 13. Ann Pharmacother. 2022. PMID: 35021923
-
A Comparison of Inhaled Epoprostenol in Patients With Acute Respiratory Distress Syndrome and COVID-19-Associated Acute Respiratory Distress Syndrome.Cureus. 2022 Aug 22;14(8):e28274. doi: 10.7759/cureus.28274. eCollection 2022 Aug. Cureus. 2022. PMID: 36158384 Free PMC article.
-
Nitric oxide versus epoprostenol for refractory hypoxemia in Covid-19.PLoS One. 2022 Jun 27;17(6):e0270646. doi: 10.1371/journal.pone.0270646. eCollection 2022. PLoS One. 2022. PMID: 35759496 Free PMC article.
-
The role of inhaled prostacyclin in treating acute respiratory distress syndrome.Ther Adv Respir Dis. 2015 Dec;9(6):302-12. doi: 10.1177/1753465815599345. Epub 2015 Aug 20. Ther Adv Respir Dis. 2015. PMID: 26294418 Review.
-
A Narrative Review on the Administration of Inhaled Prostaglandins in Critically Ill Adult Patients With Acute Respiratory Distress Syndrome.Ann Pharmacother. 2024 May;58(5):533-548. doi: 10.1177/10600280231194539. Epub 2023 Aug 17. Ann Pharmacother. 2024. PMID: 37589097 Review.
Cited by
-
Inhaled epoprostenol for management of acute respiratory failure and pulmonary vascular disease.Pulm Pharmacol Ther. 2025 Jun 11;90:102374. doi: 10.1016/j.pupt.2025.102374. Online ahead of print. Pulm Pharmacol Ther. 2025. PMID: 40513981 Review.
References
-
- van Heerden PV, Barden A, Michalopoulos N, Bulsara MK, Roberts BL. Dose-response to inhaled aerosolized prostacyclin for hypoxemia due to ARDS. Chest. 2000;117(3):819–827. - PubMed
-
- Dahlem P, van Aalderen WMC, de Neef M, Dijkgraaf MGW, Bos AP. Randomized controlled trial of aerosolized prostacyclin therapy in children with acute lung injury. Crit Care Med. 2004;32(4):1055–1060. - PubMed
-
- Hahla S, Nawal S, Salwa Z, Muniza Y, Iqbal A, Anwarul HG. Use of inhaled PGE1 to improve diastolic dysfunction, LVEDP, pulmonary hypertension and hypoxia in ARDS—a randomised clinical trial. Open J Anesthesiol. 2013;03(02):109–115.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
