Allylation of C-, N-, and O-Nucleophiles via a Mechanochemically-Driven Tsuji-Trost Reaction Suitable for Late-Stage Modification of Bioactive Molecules
- PMID: 38516646
- PMCID: PMC10953357
- DOI: 10.1002/ange.202314637
Allylation of C-, N-, and O-Nucleophiles via a Mechanochemically-Driven Tsuji-Trost Reaction Suitable for Late-Stage Modification of Bioactive Molecules
Abstract
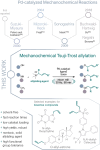
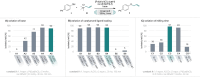
We present the first solvent-free, mechanochemical protocol for a palladium-catalyzed Tsuji-Trost allylation. This approach features exceptionally low catalyst loadings (0.5 mol %), short reaction times (<90 min), and a simple setup, eliminating the need for air or moisture precautions, making the process highly efficient and environmentally benign. We introduce solid, nontoxic, and easy-to-handle allyl trimethylammonium salts as valuable alternative to volatile or hazardous reagents. Our approach enables the allylation of various O-, N-, and C-nucleophiles in yields up to 99 % even for structurally complex bioactive compounds, owing to its mild conditions and exceptional functional group tolerance.
A mechanochemical, solvent‐free protocol for Tsuji–Trost allylation of O‐, N‐, and C‐Nucleophiles using nontoxic, solid allyl trimethylammonium chloride as alternative allylating agent is presented. This method features fast reaction kinetics, very low catalyst loadings, high yields, mild conditions, and high functional group tolerance. Its potential for late‐stage modifications of bioactive compounds is demonstrated through multiple examples.
Keywords: Ammonium Salts; Ball Milling; Green Chemistry; Mechanochemistry; Solvent Free.
© 2023 The Authors. Angewandte Chemie published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


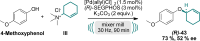
References
Grants and funding
LinkOut - more resources
Full Text Sources
