GNTI-122: an autologous antigen-specific engineered Treg cell therapy for type 1 diabetes
- PMID: 38516892
- PMCID: PMC11063937
- DOI: 10.1172/jci.insight.171844
GNTI-122: an autologous antigen-specific engineered Treg cell therapy for type 1 diabetes
Abstract
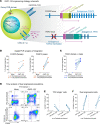
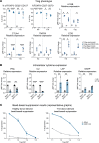
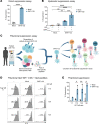
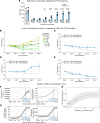
Tregs have the potential to establish long-term immune tolerance in patients recently diagnosed with type 1 diabetes (T1D) by preserving β cell function. Adoptive transfer of autologous thymic Tregs, although safe, exhibited limited efficacy in previous T1D clinical trials, likely reflecting a lack of tissue specificity, limited IL-2 signaling support, and in vivo plasticity of Tregs. Here, we report a cell engineering strategy using bulk CD4+ T cells to generate a Treg cell therapy (GNTI-122) that stably expresses FOXP3, targets the pancreas and draining lymph nodes, and incorporates a chemically inducible signaling complex (CISC). GNTI-122 cells maintained an expression profile consistent with Treg phenotype and function. Activation of CISC using rapamycin mediated concentration-dependent STAT5 phosphorylation and, in concert with T cell receptor engagement, promoted cell proliferation. In response to the cognate antigen, GNTI-122 exhibited direct and bystander suppression of polyclonal, islet-specific effector T cells from patients with T1D. In an adoptive transfer mouse model of T1D, a mouse engineered-Treg analog of GNTI-122 trafficked to the pancreas, decreased the severity of insulitis, and prevented progression to diabetes. Taken together, these findings demonstrate in vitro and in vivo activity and support further development of GNTI-122 as a potential treatment for T1D.
Keywords: Autoimmunity; Diabetes; Gene therapy; Therapeutics.
Figures
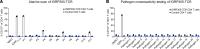


References
-
- Berdeja JG, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. doi: 10.1016/S0140-6736(21)00933-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

