A TBK1 variant causes autophagolysosomal and motoneuron pathology without neuroinflammation in mice
- PMID: 38517332
- PMCID: PMC10959724
- DOI: 10.1084/jem.20221190
A TBK1 variant causes autophagolysosomal and motoneuron pathology without neuroinflammation in mice
Abstract
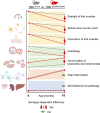
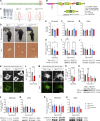
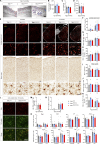
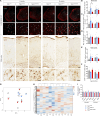
Heterozygous mutations in the TBK1 gene can cause amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). The majority of TBK1-ALS/FTD patients carry deleterious loss-of-expression mutations, and it is still unclear which TBK1 function leads to neurodegeneration. We investigated the impact of the pathogenic TBK1 missense variant p.E696K, which does not abolish protein expression, but leads to a selective loss of TBK1 binding to the autophagy adaptor protein and TBK1 substrate optineurin. Using organelle-specific proteomics, we found that in a knock-in mouse model and human iPSC-derived motor neurons, the p.E696K mutation causes presymptomatic onset of autophagolysosomal dysfunction in neurons precipitating the accumulation of damaged lysosomes. This is followed by a progressive, age-dependent motor neuron disease. Contrary to the phenotype of mice with full Tbk1 knock-out, RIPK/TNF-α-dependent hepatic, neuronal necroptosis, and overt autoinflammation were not detected. Our in vivo results indicate autophagolysosomal dysfunction as a trigger for neurodegeneration and a promising therapeutic target in TBK1-ALS/FTD.
© 2024 Brenner et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures

References
-
- Arnold, E.S., Ling S.C., Huelga S.C., Lagier-Tourenne C., Polymenidou M., Ditsworth D., Kordasiewicz H.B., McAlonis-Downes M., Platoshyn O., Parone P.A., et al. . 2013. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc. Natl. Acad. Sci. USA. 110:E736–E745. 10.1073/pnas.1222809110 - DOI - PMC - PubMed
-
- Brenner, D. 2024. Raw data and statistical analysis [Data set]. In Journal of Experimental Medicine. Version 2. Zenodo. https://doi.org/10.5281/zenodo.10511341 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

