Dynamics and prognostic value of serum neurofilament light chain in Guillain-Barré syndrome
- PMID: 38518653
- PMCID: PMC10980997
- DOI: 10.1016/j.ebiom.2024.105072
Dynamics and prognostic value of serum neurofilament light chain in Guillain-Barré syndrome
Abstract
Background: Neurofilament light chain (NfL) is a biomarker for axonal damage in several neurological disorders. We studied the longitudinal changes in serum NfL in patients with Guillain-Barré syndrome (GBS) in relation to disease severity, electrophysiological subtype, treatment response, and prognosis.
Methods: We included patients with GBS who participated in a double-blind, randomised, placebo-controlled trial that evaluated the effects of a second course of intravenous immunoglobulin (IVIg) on clinical outcomes. Serum NfL levels were measured before initiation of treatment and at one, two, four, and twelve weeks using a Simoa HD-X Analyzer. Serum NfL dynamics were analysed using linear mixed-effects models. Logistic regression was employed to determine the associations of serum NfL with clinical outcome and the prognostic value of serum NfL after correcting for known prognostic markers included in the modified Erasmus GBS Outcome Score (mEGOS).
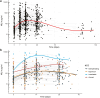
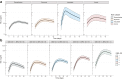
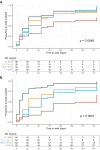
Findings: NfL levels were tested in serum from 281 patients. Serum NfL dynamics were associated with disease severity and electrophysiological subtype. Strong associations were found between high levels of serum NfL at two weeks and inability to walk unaided at four weeks (OR = 1.74, 95% CI = 1.27-2.45), and high serum NfL levels at four weeks and inability to walk unaided at 26 weeks (OR = 2.79, 95% CI = 1.72-4.90). Baseline serum NfL had the most significant prognostic value for ability to walk, independent of predictors included in the mEGOS. The time to regain ability to walk unaided was significantly longer for patients with highest serum NfL levels at baseline (p = 0.0048) and week 2 (p < 0.0001). No differences in serum NfL were observed between patients that received a second IVIg course vs. IVIg and placebo.
Interpretation: Serum NfL levels are associated with disease severity, axonal involvement, and poor outcome in GBS. Serum NfL potentially represents a biomarker to monitor neuronal damage in GBS and an intermediate endpoint to evaluate the effects of treatment.
Funding: Prinses Beatrix Spierfonds W.OR19-24.
Keywords: Biomarker; Guillain-Barré syndrome; Longitudinal; Neurofilament light chain; Prognosis.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Research of CT is supported by the European Commission (Marie Curie International Training Network, grant agreement No 860197 (MIRIADE), Innovative Medicines Initiatives 3TR (Horizon 2020, grant no 831434) EPND (IMI 2 Joint Undertaking (JU), grant No. 101034344) and JPND (bPRIDE), National MS Society (Progressive MS alliance), Alzheimer Drug Discovery Foundation, Alzheimer Association, Health Holland, the Dutch Research Council (ZonMW), The Selfridges Group Foundation, Alzheimer Netherlands. CT is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW2 (#73305095007) and Health ∼ Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). CT is recipient of TAP-dementia, a ZonMw funded project (#10510032120003) in the context of the Dutch National Dementia Strategy. CT performed contract research for ADx Neurosciences, AC-Immune, Aribio, Axon Neurosciences, Beckman–Coulter, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, Cognition Therapeutics, EIP Pharma, Eisai, Eli Lilly Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, Olink, PeopleBio, Quanterix, Roche, Siemens, Toyama, and Vivoryon. CT received payment or honoraria for lectures, presentations or educational events from Roche, Novo Nordisk and Grifols. All payments were made to her institution. CT serves on editorial boards of Medidact Neurologie/Springer; and in Neurology: Neuroimmunology & Neuroinflammation. She is editor of Alzheimer Research and Therapy. RH reports institutional funding from the GBS-CIDP Foundation International, Stichting GBS, T2B collaboration project funded by PPP Allowance made available by Top Sector Life Sciences & Health to Samenwerkende Gezondheidsfondsen (SGF) under project number LSHM18055-SGF to stimulate public-private partnerships and co-financing by health foundations that are part of the SGF, NIH, and PANDIA collaboration project co-funded by BÜHLMANN Laboratories AG and the PPP Allowance made available by Health ∼ Holland, Top Sector Life Sciences & Health, to Prinses Beatrix Spierfonds to stimulate public-private partnerships under project number LSHM23017-SGF. She serves as editorial board member of the Journal of the Peripheral Nervous System and was board member of the Inflammatory Neuropathy Consortium. PD reports grants from the Prinses Beatrix Spierfonds and Sanquin to conduct the SID-GBS trial. PD reports consulting fees from Annexon and Roche, all paid to the institution. PD participates on a Data Safety Monitoring Board or Advisory Board for Argenx, Hansa, Octapharma and Sanofi. All fees go to the institution. He reports an unpaid leadership role in the Peripheral Nerve Society (PNS) and serves on the Medical advisory board for the GBS/CIDP Foundation International. BJ reports institutional funding from the GBS-CIDP Foundation International, Stichting GBS, Horizon 2020 (EU), Grifols, CSL-Behring, Annexon, Hoffmann-la Roche, Hansa Biopharma, BÜHLMANN Laboratories. BJ received institutional royalties, licences or consulting fees from Hansa Biopharma, Annexon and Hoffmann-la Roche. BJ received support from the GBS-CIDP Foundation International to cover travel expenses for their conference. BJ serves an unpaid role in the global Medical advisory board for the GBS-CIDP Foundation International, in the scientific review committee of Stichting MS research, and the scientific review committee of the Erasmus MC. All other authors declare no competing interests.
Figures

References
-
- Willison H.J., Jacobs B.C., van Doorn P.A. Guillain-Barré syndrome. Lancet. 2016;388(10045):717–727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

