A brainstem to hypothalamic arcuate nucleus GABAergic circuit drives feeding
- PMID: 38518777
- PMCID: PMC7617324
- DOI: 10.1016/j.cub.2024.02.074
A brainstem to hypothalamic arcuate nucleus GABAergic circuit drives feeding
Abstract
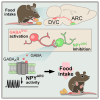
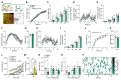
The obesity epidemic is principally driven by the consumption of more calories than the body requires. It is therefore essential that the mechanisms underpinning feeding behavior are defined. Neurons within the brainstem dorsal vagal complex (DVC) receive direct information from the digestive system and project to second-order regions in the brain to regulate food intake. Although γ-aminobutyric acid is expressed in the DVC (GABADVC), its function in this region has not been defined. In order to discover the unique gene expression signature of GABADVC cells, we used single-nucleus RNA sequencing (Nuc-seq), and this revealed 19 separate clusters. We next probed the function of GABADVC cells and discovered that the selective activation of GABADVC neurons significantly controls food intake and body weight. Optogenetic interrogation of GABADVC circuitry identified GABADVC → hypothalamic arcuate nucleus (ARC) projections as appetite suppressive without creating aversion. Electrophysiological analysis revealed that GABADVC → ARC stimulation inhibits hunger-promoting neuropeptide Y (NPY) neurons via GABA release. Adopting an intersectional genetics strategy, we clarify that the GABADVC → ARC circuit curbs food intake. These data identify GABADVC as a new modulator of feeding behavior and body weight and a controller of orexigenic NPY neuron activity, thereby providing insight into the neural underpinnings of obesity.
Keywords: body weight; dorsal vagal complex; food intake; gamma-aminobutyric acid; hunger; hypothalamus; neuropeptide Y; nucleus of the solitary tract; obesity.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

