Recent advances in vascular thiol isomerases and redox systems in platelet function and thrombosis
- PMID: 38518897
- PMCID: PMC11214884
- DOI: 10.1016/j.jtha.2024.03.008
Recent advances in vascular thiol isomerases and redox systems in platelet function and thrombosis
Abstract
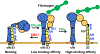
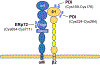
There have been substantial advances in vascular protein disulfide isomerases (PDIs) in platelet function and thrombosis in recent years. There are 4 known prothrombotic thiol isomerases; PDI, endoplasmic reticulum protein (ERp)57, ERp72, and ERp46, and 1 antithrombotic PDI; transmembrane protein 1. A sixth PDI, ERp5, may exhibit either prothrombotic or antithrombotic properties in platelets. Studies on ERp46 in platelet function and thrombosis provide insight into the mechanisms by which these enzymes function. ERp46-catalyzed disulfide cleavage in the αIIbβ3 platelet integrin occurs prior to PDI-catalyzed events to maximally support platelet aggregation. The transmembrane PDI transmembrane protein 1 counterbalances the effect of ERp46 by inhibiting activation of αIIbβ3. Recent work on the prototypic PDI found that oxidized PDI supports platelet aggregation. The a' domain of PDI is constitutively oxidized, possibly by endoplasmic reticulum oxidoreductase-1α. However, the a domain is normally reduced but becomes oxidized under conditions of oxidative stress. In contrast to the role of oxidized PDI in platelet function, reduced PDI downregulates activation of the neutrophil integrin αMβ2. Intracellular platelet PDI cooperates with Nox1 and contributes to thromboxane A2 production to support platelet function. Finally, αIIb and von Willebrand factor contain free thiols, which alter the functions of these proteins, although the extent to which the PDIs regulate these functions is unclear. We are beginning to understand the substrates and functions of vascular thiol isomerases and the redox network they form that supports hemostasis and thrombosis. Moreover, the disulfide bonds these enzymes target are being defined. The clinical implications of the knowledge gained are wide-ranging.
Keywords: disulfide; integrin; platelet; protein disulfide isomerase; sulfhydryl; thrombosis.
Copyright © 2024 International Society on Thrombosis and Haemostasis. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interests The authors declare no conflict of interest.
Figures

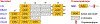
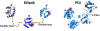

Similar articles
-
Galloylated polyphenols represent a new class of antithrombotic agents with broad activity against thiol isomerases.J Thromb Haemost. 2025 Jun;23(6):1850-1863. doi: 10.1016/j.jtha.2025.01.021. Epub 2025 Feb 12. J Thromb Haemost. 2025. PMID: 39952360
-
Multiple protein disulfide isomerases support thrombosis.Curr Opin Hematol. 2018 Sep;25(5):395-402. doi: 10.1097/MOH.0000000000000449. Curr Opin Hematol. 2018. PMID: 29994898 Free PMC article. Review.
-
Vascular thiol isomerases in thrombosis: The yin and yang.J Thromb Haemost. 2020 Nov;18(11):2790-2800. doi: 10.1111/jth.15019. Epub 2020 Aug 24. J Thromb Haemost. 2020. PMID: 32702157 Free PMC article. Review.
-
Myosin light chain 6 (Myl6) interacts with kindlin-3 and is required to support integrin αIIbβ3 activation in platelets in mice.J Thromb Haemost. 2024 Jul;22(7):2009-2017. doi: 10.1016/j.jtha.2024.01.007. Epub 2024 Jan 22. J Thromb Haemost. 2024. PMID: 38266679 Free PMC article.
-
The b' domain of protein disulfide isomerase cooperates with the a and a' domains to functionally interact with platelets.J Thromb Haemost. 2019 Feb;17(2):371-382. doi: 10.1111/jth.14366. Epub 2019 Feb 3. J Thromb Haemost. 2019. PMID: 30566278 Free PMC article.
Cited by
-
A self-sacrificing anti-inflammatory coating promotes simultaneous cardiovascular repair and reendothelialization of implanted devices.Bioact Mater. 2025 Feb 13;47:502-512. doi: 10.1016/j.bioactmat.2025.01.037. eCollection 2025 May. Bioact Mater. 2025. PMID: 40026826 Free PMC article.
-
Recent advances in vascular thiol isomerases: insights into structures, functions in thrombosis and antithrombotic inhibitor development.Thromb J. 2025 Feb 17;23(1):16. doi: 10.1186/s12959-025-00699-8. Thromb J. 2025. PMID: 39962537 Free PMC article. Review.
-
Mechanistic basis of activation and inhibition of protein disulfide isomerase by allosteric antithrombotic compounds.J Thromb Haemost. 2025 Feb;23(2):577-587. doi: 10.1016/j.jtha.2024.09.036. Epub 2024 Oct 23. J Thromb Haemost. 2025. PMID: 39454880
-
Platelets welcome a new protein disulfide isomerase family member.J Thromb Haemost. 2025 Jan;23(1):36-38. doi: 10.1016/j.jtha.2024.10.006. J Thromb Haemost. 2025. PMID: 39798968 No abstract available.
-
Galloylated polyphenols represent a new class of antithrombotic agents with broad activity against thiol isomerases.J Thromb Haemost. 2025 Jun;23(6):1850-1863. doi: 10.1016/j.jtha.2025.01.021. Epub 2025 Feb 12. J Thromb Haemost. 2025. PMID: 39952360
References
-
- von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Köllnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. The Journal of experimental medicine. 2012; 209: 819–35. 10.1084/jem.20112322. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

