Molecular assessment of intratumoral immune cell subsets and potential mechanisms of resistance to odronextamab, a CD20×CD3 bispecific antibody, in patients with relapsed/refractory B-cell non-Hodgkin lymphoma
- PMID: 38519055
- PMCID: PMC10961523
- DOI: 10.1136/jitc-2023-008338
Molecular assessment of intratumoral immune cell subsets and potential mechanisms of resistance to odronextamab, a CD20×CD3 bispecific antibody, in patients with relapsed/refractory B-cell non-Hodgkin lymphoma
Abstract
Background: Patients with relapsed/refractory B-cell non-Hodgkin lymphoma (R/R B-NHL) have a significant need for effective treatment options. Odronextamab is an Fc-silenced, human, CD20×CD3 bispecific antibody that targets CD20-expressing cells via T-cell-mediated cytotoxicity independent of T-cell/major histocompatibility complex interaction. Phase I results in patients with R/R B-NHL demonstrated that odronextamab monotherapy could achieve deep and durable responses with a generally manageable safety profile (ELM-1; NCT02290951). As part of a biomarker analysis of the same study, we investigated potential biomarkers and mechanisms of resistance to odronextamab.
Methods: Patients with R/R B-NHL enrolled in ELM-1 received one time per week doses of intravenous odronextamab for 4×21 day cycles, then doses every 2 weeks thereafter. Patient tumor biopsies were obtained at baseline, on-treatment, and at progression. Immune cell markers were analyzed by immunohistochemistry, flow cytometry, single-cell RNA sequencing, and whole genome sequencing.
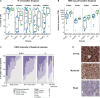
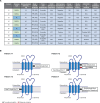
Results: Baseline tumor biopsies showed that almost all patients had high proportions of B cells that expressed the CD20 target antigen, whereas expression of other B-cell surface antigens (CD19, CD22, CD79b) was more variable. Responses to odronextamab in patients with diffuse large B-cell lymphoma were not related to the relative level of baseline CD20 expression, cell of origin, or high-risk molecular subtype. A potential link was observed between greater tumor programmed cell death-ligand 1 expression and increased likelihood of response to odronextamab. Similarly, a trend was observed between clinical response and increased levels of CD8 T cells and regulatory T cells at baseline. We also identified an on-treatment pharmacodynamic shift in intratumoral immune cell subsets. Finally, loss of CD20 expression through inactivating gene mutations was identified as a potential mechanism of resistance in patients who were treated with odronextamab until progression, as highlighted in two detailed patient cases reported here.
Conclusions: This biomarker analysis expands on clinical findings of odronextamab in patients with R/R B-NHL, providing verification of the suitability of CD20 as a therapeutic target, as well as evidence for potential mechanisms of action and resistance.
Keywords: Antibodies, Bispecific; Biomarkers, Tumor.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JB-V, NF, RPD, KJC, DS, SJ, LB, NTG, SG, CA, DMF, AC, GT, SRA, and VJ are employees of, and hold stock in, Regeneron Pharmaceuticals, Inc. MST: research funding from Regeneron Pharmaceuticals, Inc.; consulting fees from Gilead, Janssen, Kite, Novartis, Regeneron Pharmaceuticals Inc., and Roche; and support for attending meetings or travel from Amgen and Janssen. RB: employee of Ipsen; and his spouse holds stock options in and is an employee of Sanofi-Pasteur. JD: patient fees to the institution from MorphoSys. RHA: advisory board membership from ADC Therapeutics, Bristol Myers Squibb, Epizyme, Genentech, Gilead, Incyte, Karyopharm, Portola, and Seattle Genetics; and institutional research funding from Cyteir, Forty Seven, Genentech, Gilead, Janssen, Regeneron Pharmaceuticals Inc., and Roche.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
