Adverse effects of microparticles on transfusion of stored red blood cell concentrates
- PMID: 38519412
- PMCID: PMC11670589
- DOI: 10.1016/j.htct.2024.01.007
Adverse effects of microparticles on transfusion of stored red blood cell concentrates
Abstract
Background: Systemic and pulmonary coagulopathy and inflammation are important characteristics of transfusion-related acute lung injury (TRALI). Whether microparticles that accumulate in transfused red blood cell concentrates (RBCs) have proinflammatory and procoagulant potential and contribute to adverse reactions of RBC transfusions is unclear.
Aim: To investigate the ability of microparticles in stored RBCs to promote thrombin generation and induce human pulmonary microvascular endothelial cell (HMVEC) activation and damage.
Methods: The number and size of microparticles were determined by flow cytometric and nanoparticle tracking analyses, respectively. Thrombin generation and the intrinsic coagulation pathway were assayed by a calibrated automated thrombogram and by measuring activated partial thromboplastin time (aPTT), respectively. The expression of ICAM-1 and the release of cytokines by endothelial cells were detected by flow cytometric analyses. HMVEC damage was assessed by incubating lipopolysaccharide-activated endothelial cells with MP-primed polymorphonuclear neutrophils (PMNs).
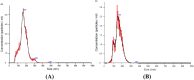
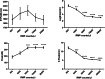
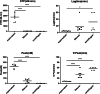
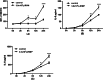
Results: The size of the microparticles in the RBC supernatant was approximately 100-300 nm. Microparticles promoted thrombin generation in a dose-dependent manner and the aPTT was shortened. Depleting microparticles from the supernatant of RBCs stored for 35 days by either filtration or centrifugation significantly decreased the promotion of thrombin generation. The expression of ICAM-1 on HMVECs was increased significantly by incubation with isolated microparticles. Furthermore, microparticles induced the release of interleukin-6 (IL-6) and interleukin-8 (IL-8) from HMVECs. Microparticles induced lipopolysaccharide-activated HMVEC damage by priming PMNs, but this effect was prevented by inhibiting the PMNs respiratory burst with apocynin.
Conclusion: Microparticles in stored RBCs promote thrombin generation, HMVEC activation and damage which may be involved in TRALI development.
Keywords: Microparticles; Procoagulant; Proinflammatory; Stored red blood cell concentrates; Transfusion-related acute lung injury.
Copyright © 2024. Published by Elsevier España, S.L.U.
Conflict of interest statement
Conflicts of interest The authors certify that they have no affiliation with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in this manuscript.
Figures


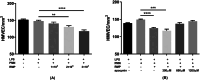
Similar articles
-
Platelet-derived microparticles induce polymorphonuclear leukocyte-mediated damage of human pulmonary microvascular endothelial cells.Transfusion. 2015 May;55(5):1051-7. doi: 10.1111/trf.12952. Epub 2015 Jan 6. Transfusion. 2015. PMID: 25565376
-
The effect of platelet-derived microparticles in stored apheresis platelet concentrates on polymorphonuclear leucocyte respiratory burst.Vox Sang. 2014 Apr;106(3):234-41. doi: 10.1111/vox.12092. Epub 2013 Oct 21. Vox Sang. 2014. PMID: 24138005
-
Microparticles in red cell concentrates prime polymorphonuclear neutrophils and cause acute lung injury in a two-event mouse model.Int Immunopharmacol. 2018 Feb;55:98-104. doi: 10.1016/j.intimp.2017.11.029. Epub 2017 Dec 22. Int Immunopharmacol. 2018. PMID: 29241160
-
Microparticles formed during storage of red blood cell units support thrombin generation.J Trauma Acute Care Surg. 2018 Apr;84(4):598-605. doi: 10.1097/TA.0000000000001759. J Trauma Acute Care Surg. 2018. PMID: 29251713 Free PMC article.
-
Extracellular Vesicles in Red Blood Cell Concentrates: An Overview.Transfus Med Rev. 2019 Apr;33(2):125-130. doi: 10.1016/j.tmrv.2019.02.002. Epub 2019 Feb 23. Transfus Med Rev. 2019. PMID: 30910256 Review.
Cited by
-
Transfusion-Related Acute Lung Injury: from Mechanistic Insights to Therapeutic Strategies.Adv Sci (Weinh). 2025 Mar;12(11):e2413364. doi: 10.1002/advs.202413364. Epub 2025 Jan 21. Adv Sci (Weinh). 2025. PMID: 39836498 Free PMC article. Review.
References
-
- Adeline W., Bérangère D., Bernard C., Christian C., Jean-Michel D., François M. Extracellular vesicles in red blood cell concentrates: an overview. Transfus Med Rev. 2019 Apr;33(2):125–130. - PubMed
-
- Almizraq R., Tchir J.D., Holovati J.L., Acker J.P. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013;53:2258–2267. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

