Once-weekly versus twice-weekly bortezomib in newly diagnosed multiple myeloma: a real-world analysis
- PMID: 38519476
- PMCID: PMC10959949
- DOI: 10.1038/s41408-024-01034-6
Once-weekly versus twice-weekly bortezomib in newly diagnosed multiple myeloma: a real-world analysis
Abstract
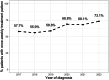
Induction regimens for multiple myeloma (MM) commonly include bortezomib, which has typically been administered twice weekly despite studies demonstrating comparable efficacy and less peripheral neuropathy (PN) with once-weekly bortezomib. We aimed to analyze the real-world prevalence and efficacy of once-weekly versus twice-weekly bortezomib regimens in newly diagnosed MM. We analyzed 2497 US patients aged 18-70 years treated with commercial first-line bortezomib using nationwide Flatiron Health electronic health record-derived data, including 910 (36.4%) patients who received twice-weekly and 1522 (63.2%) who received once-weekly bortezomib. Once-weekly bortezomib use increased over time, from 57.7% in 2017 to 73.1% in 2022. Multivariate analysis identified worsened performance status and more recent year of diagnosis with higher odds of receiving once-weekly bortezomib. Real-world progression-free survival (median 37.2 months with once-weekly versus 39.6 months with twice-weekly, p = 0.906) and overall survival (medians not reached in either cohort, p = 0.800) were comparable. PN rates were higher in patients receiving twice-weekly bortezomib (34.7% versus 18.5%, p < 0.001). In conclusion, once-weekly bortezomib is clearly associated with similar efficacy and fewer toxicities compared to twice-weekly bortezomib. Our findings support once-weekly bortezomib as a standard-of-care regimen for newly diagnosed patients with MM.
© 2024. The Author(s).
Conflict of interest statement
RB: Consulting: BMS, Caribou Biosciences, Genentech, Janssen, Karyopharm, Pfizer, Sanofi, SparkCures; Research: Pack Health. XW: Employment: Flatiron Health, Inc. (independent subsidiary of the Roche Group), stock ownership, Roche. JR: Employment: Flatiron Health, Inc. (independent subsidiary of the Roche Group), stock ownership, Roche. LDA Jr: Consulting: Janssen, Celgene, BMS, Amgen, GSK, AbbVie, Beigene, Cellectar, Sanofi, Prothena. Research: BMS, Celgene, GSK, Janssen, Abbvie. AJC: Consulting: BMS, Adaptive; Research: Adaptive Biotechnologies, Harpoon, Nektar, BMS, Janssen, Sanofi, AbbVie. GK: Consulting: BMS, Arcellx, Sanofi, Janssen, Cellectar, Pfizer, Kedrion; Research: BMS, Janssen, Abbvie. The remaining authors have no disclosures to report.
Figures


References
-
- Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–27. doi: 10.1016/S0140-6736(16)31594-X. - DOI - PMC - PubMed
-
- Joseph NS, Kaufman JL, Dhodapkar MV, Hofmeister CC, Almaula DK, Heffner LT, et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020;38:1928–37. doi: 10.1200/JCO.19.02515. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

