Lactobacillus casei Shirota probiotic drinks reduce antibiotic associated diarrhoea in patients with spinal cord injuries who regularly consume proton pump inhibitors: a subgroup analysis of the ECLISP multicentre RCT
- PMID: 38519563
- PMCID: PMC11176055
- DOI: 10.1038/s41393-024-00983-w
Lactobacillus casei Shirota probiotic drinks reduce antibiotic associated diarrhoea in patients with spinal cord injuries who regularly consume proton pump inhibitors: a subgroup analysis of the ECLISP multicentre RCT
Abstract
Study design: This was a sub-group analysis of a multicentre, randomised, placebo-controlled, double-blind trial (ECLISP trial) OBJECTIVES: To assess the efficacy of a probiotic containing at least 6.5 × 109 live Lactobacillus casei Shirota (LcS) in preventing antibiotic associated diarrhoea (AAD) in patients with spinal cord injury (SCI) who consumed proton pump inhibitor (PPI) regularly. LcS or placebo was given once daily for the duration of an antibiotic course and continued for 7 days thereafter. The trial was registered with ISRCTN:13119162.
Setting: Three SCI centres (National Spinal Injuries Centre, Midland Centre for Spinal Injuries and Princess Royal Spinal Cord Injuries Centre) in the United Kingdom METHODS: Between November 2014, and November 2019, 95 eligible consenting SCI patients (median age: 57; IQ range: 43-69) were randomly allocated to receive LcS (n = 50) or placebo (n = 45). The primary outcome is the occurrence of AAD up to 30 days after finishing LcS/placebo.
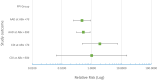
Results: The LcS group had a significantly lower incidence of AAD at 30 days after finishing the antibiotic course (28.0 v 53.3%, RR: 95% CI: 0.53, 0.31-0.89; z = 2.5, p = 0.01). Multivariate logistic regression analysis identified that LcS can reduce the risk of AAD at 30 days (OR: 0.36, 95% CI 0.13, 0.99, p < 0.05). No intervention-related adverse events were reported during the study.
Conclusions: LcS has the potential to prevent AAD in what could be considered a defined vulnerable group of SCI patients on regular PPI. A confirmatory, randomised, placebo-controlled study is needed to confirm this apparent therapeutic success to translate it into appropriate clinical outcomes.
Sponsorship: Yakult Honsha Co., Ltd.
© 2024. Crown.
Conflict of interest statement
SW has received funding support from Yakult Honsha Co Ltd. Yakult Europe B.V. provided free LcS/Placebo and the necessary equipment to maintain the cold chain. The company was aware of, but did not influence the trial design, and had no role in data analysis and interpretation. AF has received honoraria form Fresenius Kabi, Takeda and Dr Falk Pharma. SW as chief investigator and corresponding author had full access to the final dataset and made final decision to submit for publication.
Figures
References
-
- Spinal cord injury paralyses someone every four hours, new estimates reveal. Spinal Injury Association. 2022. https://www.spinal.co.uk/news/spinal-cord-injury-paralyses-someone-every.... Accessed 16th January 2023.
-
- Wong S, Santullo P, Hirani SP, Kumar N, Chowdhury JR, Carcia-Forcada A, et al. Use of antibiotic and the prevalence of antibiotic-associated dirrhoea in patients with spinal cord injuries: an international, multi-centre study. J Hosp Infect. 2017;97:146–52. doi: 10.1016/j.jhin.2017.06.019. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical