Platinum iodido drugs show potential anti-tumor activity, affecting cancer cell metabolism and inducing ROS and senescence in gastrointestinal cancer cells
- PMID: 38519773
- PMCID: PMC10959927
- DOI: 10.1038/s42003-024-06052-5
Platinum iodido drugs show potential anti-tumor activity, affecting cancer cell metabolism and inducing ROS and senescence in gastrointestinal cancer cells
Abstract
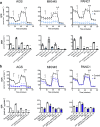
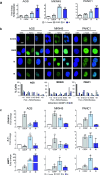
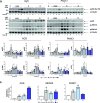
Cisplatin-based chemotherapy has associated clinical disadvantages, such as high toxicity and resistance. Thus, the development of new antitumor metallodrugs able to overcome different clinical barriers is a public healthcare priority. Here, we studied the mechanism of action of the isomers trans and cis-[PtI2(isopropylamine)2] (I5 and I6, respectively) against gastrointestinal cancer cells. We demonstrate that I5 and I6 modulate mitochondrial metabolism, decreasing OXPHOS activity and negatively affecting ATP-linked oxygen consumption rate. Consequently, I5 and I6 generated Reactive Oxygen Species (ROS), provoking oxidative damage and eventually the induction of senescence. Thus, herein we propose a loop with three interconnected processes modulated by these iodido agents: (i) mitochondrial dysfunction and metabolic disruptions; (ii) ROS generation and oxidative damage; and (iii) cellular senescence. Functionally, I5 reduces cancer cell clonogenicity and tumor growth in a pancreatic xenograft model without systemic toxicity, highlighting a potential anticancer complex that warrants additional pre-clinical studies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
A novel inhibitor of the mitochondrial respiratory complex I with uncoupling properties exerts potent antitumor activity.Cell Death Dis. 2024 May 2;15(5):311. doi: 10.1038/s41419-024-06668-9. Cell Death Dis. 2024. PMID: 38697987 Free PMC article.
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
-
Inhibition of palmitoyltransferase ZDHHC12 sensitizes ovarian cancer cells to cisplatin through ROS-mediated mechanisms.Cancer Sci. 2024 Apr;115(4):1170-1183. doi: 10.1111/cas.16085. Epub 2024 Jan 29. Cancer Sci. 2024. PMID: 38287874 Free PMC article.
-
A systematic overview of chemotherapy effects in ovarian cancer.Acta Oncol. 2001;40(2-3):340-60. doi: 10.1080/02841860151116420. Acta Oncol. 2001. PMID: 11441940
-
Downregulation of LATS1/2 Drives Endothelial Senescence-Associated Stemness (SAS) and Atherothrombotic Lesion Formation.bioRxiv [Preprint]. 2025 Jun 21:2025.06.19.660635. doi: 10.1101/2025.06.19.660635. bioRxiv. 2025. PMID: 40667385 Free PMC article. Preprint.
Cited by
-
Trans-[Pt(amine)Cl2(PPh3)] Complexes Target Mitochondria and Endoplasmic Reticulum in Gastric Cancer Cells.Int J Mol Sci. 2024 Jul 15;25(14):7739. doi: 10.3390/ijms25147739. Int J Mol Sci. 2024. PMID: 39062981 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

