A comparison of sodium-glucose co-transporter 2 inhibitor kidney outcome trial participants with a real-world chronic kidney disease primary care population
- PMID: 38520170
- PMCID: PMC11852264
- DOI: 10.1093/ndt/gfae071
A comparison of sodium-glucose co-transporter 2 inhibitor kidney outcome trial participants with a real-world chronic kidney disease primary care population
Abstract
Background: Observational studies suggest sodium-glucose co-transporter 2 (SGLT2) inhibitor kidney outcome trials are not representative of the broader population of people with chronic kidney disease (CKD). However, there are limited data on the generalizability to those without co-existing type 2 diabetes (T2D), and the representativeness of the Study of Heart and Kidney Protection with Empagliflozin (EMPA-KIDNEY) trial has not been adequately explored. We hypothesized that SGLT2 inhibitor kidney outcome trials are more representative of people with co-existing T2D than those without, and that EMPA-KIDNEY is more representative than previous trials.
Methods: A cross-sectional analysis of adults with CKD in English primary care was conducted using the Oxford-Royal College of General Practitioners Clinical Informatics Digital Hub. The proportions that met the eligibility criteria of SGLT2 inhibitor kidney outcome trials were determined, and their characteristics described. Logistic regression analyses were performed to identify factors associated with trial eligibility.
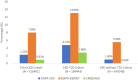
Results: Of 6 670 829 adults, 516 491 (7.7%) with CKD were identified. In the real-world CKD population, 0.9%, 2.2% and 8.0% met the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), Dapagliflozin and Renal Outcomes and Cardiovascular Mortality in Patients with Chronic Kidney Disease (DAPA-CKD) and EMPA-KIDNEY eligibility criteria, respectively. All trials were more representative of people with co-existing T2D than those without T2D. Trial participants were 9-14 years younger than the real-world CKD population, and had more advanced CKD, including higher levels of albuminuria. A higher proportion of the CREDENCE (100%), DAPA-CKD (67.6%) and EMPA-KIDNEY (44.5%) trial participants had T2D compared with the real-world CKD population (32.8%). Renin-angiotensin system inhibitors were prescribed in almost all trial participants, compared with less than half of the real-world CKD population. Females were under-represented and less likely to be eligible for the trials.
Conclusion: SGLT2 inhibitor kidney outcome trials represent a subgroup of people with CKD at high risk of adverse kidney events. Our study highlights the importance of complementing trials with real-world studies, exploring the effectiveness of SGLT2 inhibitors in the broader population of people with CKD.
Keywords: chronic kidney failure; computerized medical records systems; cross-sectional studies; primary health care; sodium-glucose transporter 2 inhibitors.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

