Development of a next generation SNP genotyping array for wheat
- PMID: 38520342
- PMCID: PMC11258986
- DOI: 10.1111/pbi.14341
Development of a next generation SNP genotyping array for wheat
Abstract
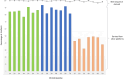
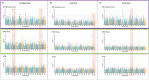
High-throughput genotyping arrays have provided a cost-effective, reliable and interoperable system for genotyping hexaploid wheat and its relatives. Existing, highly cited arrays including our 35K Wheat Breeder's array and the Illumina 90K array were designed based on a limited amount of varietal sequence diversity and with imperfect knowledge of SNP positions. Recent progress in wheat sequencing has given us access to a vast pool of SNP diversity, whilst technological improvements have allowed us to fit significantly more probes onto a 384-well format Axiom array than previously possible. Here we describe a novel Axiom genotyping array, the 'Triticum aestivum Next Generation' array (TaNG), largely derived from whole genome skim sequencing of 204 elite wheat lines and 111 wheat landraces taken from the Watkins 'Core Collection'. We used a novel haplotype optimization approach to select SNPs with the highest combined varietal discrimination and a design iteration step to test and replace SNPs which failed to convert to reliable markers. The final design with 43 372 SNPs contains a combination of haplotype-optimized novel SNPs and legacy cross-platform markers. We show that this design has an improved distribution of SNPs compared to previous arrays and can be used to generate genetic maps with a significantly higher number of distinct bins than our previous array. We also demonstrate the improved performance of TaNGv1.1 for Genome-wide association studies (GWAS) and its utility for Copy Number Variation (CNV) analysis. The array is commercially available with supporting marker annotations and initial genotyping results freely available.
Keywords: Triticum aestivum; Axiom array; breeding; genotyping; single nucleotide polymorphism; wheat.
© 2024 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
IS and RBA work for Thermo Fisher Scientific. All other authors declare no conflict of interests.
Figures





References
-
- Allen, A.M. , Winfield, M.O. , Burridge, A.J. , Downie, R.C. , Benbow, H.R. , Barker, G.L. , Wilkinson, P.A. et al. (2017) Characterization of a Wheat Breeders' Array suitable for high‐throughput SNP genotyping of global accessions of hexaploid bread wheat (Triticum aestivum). Plant Biotechnol. J. 15, 390–401. - PMC - PubMed
-
- Arruda, M.P. , Lipka, A.E. , Brown, P.J. , Krill, A.M. , Thurber, C. , Brown‐Guedira, G. , Dong, Y. et al. (2016) Comparing genomic selection and marker‐assisted selection for Fusarium head blight resistance in wheat (Triticum aestivum L.). Mol. Breed. 36, 84.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

