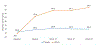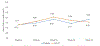High-dose vitamin D to attenuate bone loss in patients with prostate cancer on androgen deprivation therapy: A phase 2 RCT
- PMID: 38520382
- PMCID: PMC11214601
- DOI: 10.1002/cncr.35275
High-dose vitamin D to attenuate bone loss in patients with prostate cancer on androgen deprivation therapy: A phase 2 RCT
Abstract
Background: Androgen deprivation therapy (ADT) inhibits prostate cancer growth. However, ADT causes loss of bone mineral density (BMD) and an increase in fracture risk; effective interventions for ADT-induced bone loss are limited.
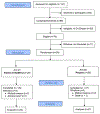
Methods: A phase 2 randomized controlled trial investigated the feasibility, safety, and preliminary efficacy of high-dose weekly vitamin D (HDVD, 50,000 IU/week) versus placebo for 24 weeks in patients with prostate cancer receiving ADT, with all subjects receiving 600 IU/day vitamin D and 1000 mg/day calcium. Participants were ≥60 years (mean years, 67.7), had a serum 25-hydroxyvitamin D level <32 ng/mL, and initiated ADT within the previous 6 months. At baseline and after intervention, dual-energy x-ray absorptiometry was used to assess BMD, and levels of bone cell, bone formation, and resorption were measured.
Results: The HDVD group (N = 29) lost 1.5% BMD at the total hip vs. 4.1% for the low-dose group (N = 30; p = .03) and 1.7% BMD at the femoral neck vs. 4.4% in the low-dose group (p = .06). Stratified analyses showed that, for those with baseline 25-hydroxyvitamin D level <27 ng/mL, the HDVD group lost 2.3% BMD at the total hip vs 7.1% for the low-dose group (p < .01). Those in the HDVD arm showed significant changes in parathyroid hormone (p < .01), osteoprotegerin (p < 0.01), N-terminal telopeptide of type 1 collagen (p < 0.01) and C-terminal telopeptide of type 1 collagen (p < 0.01). No difference in adverse events or toxicity was noted between the groups.
Conclusions: HDVD supplementation significantly reduced hip and femoral neck BMD loss, especially for patients with low baseline serum 25-hydroxyvitamin D levels, although demonstrating safety and feasibility in prostate cancer patients on ADT.
Keywords: adverse effects; hormonal therapy; osteoporosis; prostatic neoplasms; vitamin D; vitamins.
© 2024 American Cancer Society.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or competitors.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical