The Effect of Levetiracetam Compared with Enzyme-Inducing Antiseizure Medications on Apixaban and Rivaroxaban Peak Plasma Concentrations
- PMID: 38520503
- PMCID: PMC11026229
- DOI: 10.1007/s40263-024-01077-0
The Effect of Levetiracetam Compared with Enzyme-Inducing Antiseizure Medications on Apixaban and Rivaroxaban Peak Plasma Concentrations
Abstract
Background and objective: Post-stroke epilepsy represents an important clinical challenge as it often requires both treatment with direct oral anticoagulants (DOACs) and antiseizure medications (ASMs). Levetiracetam (LEV), an ASM not known to induce metabolizing enzymes, has been suggested as a safer alternative to enzyme-inducing (EI)-ASMs in patients treated with DOACs; however, current clinical guidelines suggest caution when LEV is used with DOACs because of possible P-glycoprotein induction and competition (based on preclinical studies). We investigated whether LEV affects apixaban and rivaroxaban concentrations compared with two control groups: (a) patients treated with EI-ASMs and (b) patients not treated with any ASM.
Methods: In this retrospective observational study, we monitored apixaban and rivaroxaban peak plasma concentrations (Cmax) in 203 patients treated with LEV (n = 28) and with EI-ASM (n = 33), and in patients not treated with any ASM (n = 142). Enzyme-inducing ASMs included carbamazepine, phenytoin, phenobarbital, primidone, and oxcarbazepine. We collected clinical and laboratory data for analysis, and DOAC Cmax of patients taking LEV were compared with the other two groups.
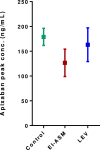
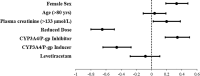
Results: In 203 patients, 55% were female and the mean age was 78 ± 0.8 years. One hundred and eighty-six patients received apixaban and 17 patients received rivaroxaban. The proportion of patients with DOAC Cmax below their therapeutic range was 7.1% in the LEV group, 10.6% in the non-ASM group, and 36.4% in the EI-ASM group (p < 0.001). The odds of having DOAC Cmax below the therapeutic range (compared with control groups) was not significantly different in patients taking LEV (adjusted odds ratio 0.70, 95% confidence interval 0.19-2.67, p = 0.61), but it was 12.7-fold higher in patients taking EI-ASM (p < 0.001). In an analysis in patients treated with apixaban, there was no difference in apixaban Cmax between patients treated with LEV and non-ASM controls, and LEV clinical use was not associated with variability in apixaban Cmax in a multivariate linear regression.
Conclusions: In this study, we show that unlike EI-ASMs, LEV clinical use was not significantly associated with lower apixaban Cmax and was similar to that in patients not treated with any ASM. Our findings suggest that the combination of LEV with apixaban and rivaroxaban may not be associated with decreased apixaban and rivaroxaban Cmax. Therefore, prospective controlled studies are required to examine the possible non-pharmacokinetic mechanism of the effect of the LEV-apixaban or LEV-rivaroxaban combination on patients' outcomes.
© 2024. The Author(s).
Conflict of interest statement
Meir Bialer received speaker’s or consultancy fees from Clexio Bioscines, Guidepoint, Meditec (Sam-On,) Pharma Two B, Selene Therapeurics, Shackelford Pharamad, USWorldMeds, and Xenon Pharma. Mordechai Muszkat recieved honorarai from Roche and a research grant from the Pfizer Independent Galobal Medical Grant. Rachel Goldstein, Natalie Rabkin, Noa Buchman, Aviya R. Jacobs, Khaled Sandouka, Bruria Raccah, Tamar Fisher Negev, and Ilan Matok have no conflicts of interest that are directly relevant to the content of this article.
Figures
References
-
- US Food and Drug Administration. Keppra prescribing information. 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021035s104,021.... Accessed 25 Jun 2023.
-
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

