Formation of potentially toxic metabolites of drugs in reactions catalyzed by human drug-metabolizing enzymes
- PMID: 38520539
- PMCID: PMC11539061
- DOI: 10.1007/s00204-024-03710-9
Formation of potentially toxic metabolites of drugs in reactions catalyzed by human drug-metabolizing enzymes
Abstract
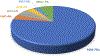
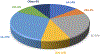
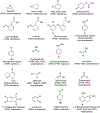
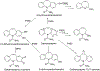
Data are presented on the formation of potentially toxic metabolites of drugs that are substrates of human drug metabolizing enzymes. The tabular data lists the formation of potentially toxic/reactive products. The data were obtained from in vitro experiments and showed that the oxidative reactions predominate (with 96% of the total potential toxication reactions). Reductive reactions (e.g., reduction of nitro to amino group and reductive dehalogenation) participate to the extent of 4%. Of the enzymes, cytochrome P450 (P450, CYP) enzymes catalyzed 72% of the reactions, myeloperoxidase (MPO) 7%, flavin-containing monooxygenase (FMO) 3%, aldehyde oxidase (AOX) 4%, sulfotransferase (SULT) 5%, and a group of minor participating enzymes to the extent of 9%. Within the P450 Superfamily, P450 Subfamily 3A (P450 3A4 and 3A5) participates to the extent of 27% and the Subfamily 2C (P450 2C9 and P450 2C19) to the extent of 16%, together catalyzing 43% of the reactions, followed by P450 Subfamily 1A (P450 1A1 and P450 1A2) with 15%. The P450 2D6 enzyme participated in an extent of 8%, P450 2E1 in 10%, and P450 2B6 in 6% of the reactions. All other enzymes participate to the extent of 14%. The data show that, of the human enzymes analyzed, P450 enzymes were dominant in catalyzing potential toxication reactions of drugs and their metabolites, with the major role assigned to the P450 Subfamily 3A and significant participation of the P450 Subfamilies 2C and 1A, plus the 2D6, 2E1 and 2B6 enzymes contributing. Selected examples of drugs that are activated or proposed to form toxic species are discussed.
Keywords: Drugs; Human enzymes; Reactive species; Toxic metabolites; Toxicity impact.
© 2024. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures
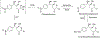



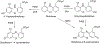
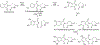
Similar articles
-
Human Family 1-4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: an update.Arch Toxicol. 2021 Feb;95(2):395-472. doi: 10.1007/s00204-020-02971-4. Epub 2021 Jan 18. Arch Toxicol. 2021. PMID: 33459808 Free PMC article. Review.
-
Cytochrome P450-mediated metabolism of haloperidol and reduced haloperidol to pyridinium metabolites.Chem Res Toxicol. 2006 Jul;19(7):914-20. doi: 10.1021/tx0600090. Chem Res Toxicol. 2006. PMID: 16841959
-
In vitro sulfoxidation of thioether compounds by human cytochrome P450 and flavin-containing monooxygenase isoforms with particular reference to the CYP2C subfamily.Drug Metab Dispos. 2004 Mar;32(3):333-9. doi: 10.1124/dmd.32.3.333. Drug Metab Dispos. 2004. PMID: 14977868
-
Metabolism and interactions of Ivermectin with human cytochrome P450 enzymes and drug transporters, possible adverse and toxic effects.Arch Toxicol. 2021 May;95(5):1535-1546. doi: 10.1007/s00204-021-03025-z. Epub 2021 Mar 15. Arch Toxicol. 2021. PMID: 33719007 Free PMC article. Review.
-
Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians.J Pharmacol Exp Ther. 1994 Jul;270(1):414-23. J Pharmacol Exp Ther. 1994. PMID: 8035341
Cited by
-
Mono-CYP CHO Model: A Recombinant Chinese Hamster Ovary Cell Platform for Investigating CYP-Specific Tamoxifen Metabolism.Int J Mol Sci. 2025 Apr 23;26(9):3992. doi: 10.3390/ijms26093992. Int J Mol Sci. 2025. PMID: 40362231 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

