Real-World Safety Outcomes with Brolucizumab in Neovascular Age-Related Macular Degeneration: Findings from the IRIS® Registry
- PMID: 38520643
- PMCID: PMC11039576
- DOI: 10.1007/s40123-024-00920-3
Real-World Safety Outcomes with Brolucizumab in Neovascular Age-Related Macular Degeneration: Findings from the IRIS® Registry
Abstract
Introduction: To assess real-world safety outcomes for adults with neovascular age-related macular degeneration (nAMD) treated with brolucizumab from the US-based IRIS® (Intelligent Research in Sight) Registry.
Methods: In this retrospective study, 18,312 eyes (15,998 patients) treated with ≥ 1 intravitreal brolucizumab injections between 8 October 2019 (US launch date for brolucizumab) and 7 October 2021 were followed up for ≤ 2 years after first injection (index date). The study assessed the predefined incident ocular adverse events of intraocular inflammation (IOI), retinal vasculitis (RV), and retinal vascular occlusion (RO).
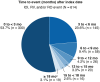
Results: Overall, 614/18,312 eyes (3.4%) experienced any IOI, RV, and/or RO event. Median (interquartile range [IQR]) time to an event was 84 (42-167) days; 77.4% of events (475/614) occurred within 6 months after index date. Median (IQR) number of brolucizumab injections before an event was 2 (1-4). For eyes with an adverse event and visual acuity (VA) data (n = 406), median (IQR) change in Early Treatment of Diabetic Retinopathy Study (ETDRS) letters from pre-event VA was 0 (- 7 to + 5) at the 6-month follow-up; 50 eyes (12.3%) had a VA loss of 10 or more ETDRS letters. Risk of an event (hazard ratio [95% confidence interval]) was decreased in eyes from male patients (0.61 [0.53-0.71]), from older patients (0.83 [0.76-0.90]), from treatment-naive patients (0.51 [0.38-0.69]), and from patients who started brolucizumab in the second year after launch (0.68 [0.53-0.86] vs. first year).
Conclusion: In this large real-world brolucizumab safety study, 3.4% of eyes experienced an IOI, RV, and/or RO event. Among eyes that experienced an adverse event for which VA data were available, median ETDRS vision change was 0 letters (IQR - 7 to + 5).
Keywords: Anti-vascular endothelial growth factor therapy; Brolucizumab; Intraocular inflammation; Neovascular age-related macular degeneration; Real-world evidence.
© 2024. The Author(s).
Conflict of interest statement
Marco A. Zarbin: Consultant for Boehringer Ingelheim, EdiGene, Genentech, Inc./Roche, Illuminare, Life Biosciences, Novartis, Perfuse Therapeutics, Seeing Medicines, Smile Biotech, Tamarix Pharmaceuticals, and Tenpoint Therapeutics; holds stock in NVasc. Mathew W. MacCumber: Consultant for Alimera Sciences, Inc., Bausch + Lomb, Genentech, Inc./Roche, IVERIC bio, Novartis Pharma AG, Regeneron Pharmaceuticals, Inc.; research grants from Alimera Sciences, Inc., Apellis Pharmaceutical, Inc., REGENXBIO. Helene Karcher: employee and shareholder of Novartis. Eser Adiguzel: employee and shareholder of Novartis. Andrew Mayhook: contracted employee of Oxford PharmaGenesis. Andrew LaPrise: employee of Verana Health, Inc. Ver L. Bilano: employee of Novartis. Franklin Igwe: employee and shareholder of Novartis. Michael S. Ip: Consultant for Alimera, Allergan, Amgen, Apellis, Clearside Biomedical, Genentech, IVERIC bio, Novartis, Regeneron, and REGENXBIO; research support from 4DMT, Apellis, Biogen, Genentech, IVERIC bio, Lineage Cell Therapeutics, ONL Therapeutics, and REGENXBIO. Charles C. Wykoff: Consultant for 4DMT, AbbVie, Adverum Biotechnologies, Aerie, AGTC, Alcon, Alimera, Allergan, Allgenesis, Alnylam, Annexon Biosciences, Apellis, Arrowhead, Ascidian, Bausch + Lomb, Bayer, Bionic Vision Technologies, Boehringer Ingelheim, Cholgene, Clearside Biomedical, Curacle, Eyebiotech, EyePoint Pharmaceuticals, Foresite, Frontera Therapeutics, Genentech, Gyroscope Therapeutics, IACTA, IVERIC bio, Janssen, Kato Pharma, Kiora, Kodiak Sciences, Kriya Therapeutics, Merck, Nanoscope, Neurotech, NGM Biopharmaceuticals, Notal Vision, Novartis, OccuRx, Ocular Therapeutix, Ocuphire, OcuTerra, OliX, ONL, Opthea, Oxular, Palatin Technologies, Perceive Bio, Perfuse, PolyPhotonix, Ray, RecensMedical, Regeneron, REGENXBIO, Resonance, Roche, Sandoz, Sanofi, SciNeuro Pharmaceuticals, Stealth Biotherapeutics, Surrozen, Suzhou Raymon, Takeda, Thea, Therini, TissueGen, Valo, and Verana Health; research funding from 4DMT, Adverum Biotechnologies, AffaMed Therapeutics, Aldeyra, Alexion, Alimera, Alkahest, Allergan, Allgenesis, Amgen, Annexin Pharmaceuticals, Annexon Biosciences, Apellis, AsclepiX Therapeutics, Bayer, Boehringer Ingelheim, Chengdu Kanghong, Clearside Biomedical, Curacle, Eyebiotech, EyePoint Pharmaceuticals, Gemini, Genentech, GlaxoSmithKline, Graybug Vision, Gyroscope Therapeutics, IONIS, iRENIX, IVERIC bio, Janssen, Kodiak Sciences, LMRI, McMaster University, Nanoscope, Neurotech, NGM Biopharmaceuticals, Novartis, Ocular Therapeutix, Ocuphire, OcuTerra, OliX, Ophthotech, Opthea, Oxurion, Oxular, Oyster Point Pharma, Perceive Bio, RecensMedical, Regeneron, REGENXBIO, Rezolute, Roche, Sam Chun Dang Pharm, Sandoz, Senju Pharmaceutical, Shanghai Henlius Biotech, Taiwan Liposome Company, Unity Biotechnology, Verily Life Sciences, and Xbrane Biopharma; on Advisory board of Aerie, Kato Pharma;. Board member of ASRS, Vit-Buckle Society; stock options for ONL, PolyPhotonix, RecensMedical, TissueGen, Visgenx, Vitranu.
Figures

References
-
- Novartis AG. Beovu (brolucizumab) safety information: overview. https://www.brolucizumab.info/overview Accessed 6 Feb 2023.
LinkOut - more resources
Full Text Sources
Research Materials

