Structural Changes in the Carbon Sphere of a Dirhodium Complex Induced by Redox or Deprotonation Reactions
- PMID: 38520714
- PMCID: PMC11165463
- DOI: 10.1002/advs.202400072
Structural Changes in the Carbon Sphere of a Dirhodium Complex Induced by Redox or Deprotonation Reactions
Abstract
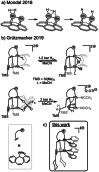
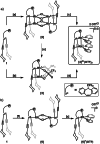
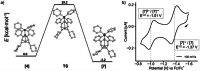
A carbon-rich molecule is synthesized, which mainly contains conjugated sp2 and sp hybridized carbon centers. Alkenyl and alkynyl binding sites are arranged such that this compound serves as ligand to a binuclear metal unit with a RhI─RhI bond. Furthermore, CH units are placed in proximity to the metal centers. The dicationic complex [Rh2(bipy)2{Ph2Ptrop(C≡CCy)2}]2+(OTf-)2 allows to study possible responses of the carbon-framework to redox reactions as well as deprotonation reactions. All products are, whenever possible, characterized by X-ray diffraction (XRD) methods, NMR and EPR spectroscopy as well as electrochemical methods. It is shown that the carbon skeleton of the ligand framework undergoes C─C bond rearrangement reactions of remarkable diversity. In combination with DFT (density functional theory) studies, these results allow to gain insight into the electronic structure changes caused by metal sites in a carbon-rich environment, which may be of relevance for the properties of metal particles on carbon support materials when they are exposed to hydrogen, electrons, or protons.
Keywords: alkynyl complexes; dinuclear complexes; low‐valent metals; metal‐metal bonds; rearrangements; redox chemistry; rhodium.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

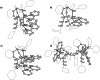
Similar articles
-
Transition Metal-Catalyzed Regioselective Direct C-H Amidation: Interplay between Inner- and Outer-Sphere Pathways for Nitrene Cross-Coupling Reactions.Acc Chem Res. 2022 Aug 2;55(15):2123-2137. doi: 10.1021/acs.accounts.2c00283. Epub 2022 Jul 19. Acc Chem Res. 2022. PMID: 35853135
-
Three Reversible Redox States of Thiolate-Bridged Dirhodium Complexes without Metal-Metal Bonds.J Am Chem Soc. 2020 Sep 23;142(38):16313-16323. doi: 10.1021/jacs.0c06205. Epub 2020 Sep 9. J Am Chem Soc. 2020. PMID: 32790995
-
Oxidative-Addition Reactions of Diiodine to Dinuclear Rhodium Pyrazolate Complexes.Inorg Chem. 1999 Mar 22;38(6):1108-1117. doi: 10.1021/ic980650s. Inorg Chem. 1999. PMID: 11670891
-
Electronic and Steric Tuning of a Prototypical Piano Stool Complex: Rh(III) Catalysis for C-H Functionalization.Acc Chem Res. 2018 Jan 16;51(1):170-180. doi: 10.1021/acs.accounts.7b00444. Epub 2017 Dec 22. Acc Chem Res. 2018. PMID: 29272106 Free PMC article. Review.
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
References
-
- Trogler W. C., Organometallic Radical Processes, De Gruyter, Berlin: 1990.
-
- Astruc D., Electron Transfer and Radical Processes in Transition‐Metal Chemistry, John Wiley & Sons, New York: 1995.
-
- Geiger W. E., Organometallics 2007, 26, 5738.
-
- Connelly N. G., in Molecular Electrochemistry of Inorganic, Bioinorganic and Organometallic Compounds, Springer Netherlands, Dordrecht, 1993, pp. 317–329.
-
- Valyaev D. A., Semeikin O. V., Ustynyuk N. A., Coord. Chem. Rev. 2004, 248, 1679.
Grants and funding
LinkOut - more resources
Full Text Sources
