Design of PD-L1-Targeted Lipid Nanoparticles to Turn on PTEN for Efficient Cancer Therapy
- PMID: 38520717
- PMCID: PMC11165541
- DOI: 10.1002/advs.202309917
Design of PD-L1-Targeted Lipid Nanoparticles to Turn on PTEN for Efficient Cancer Therapy
Abstract
Lipid nanoparticles (LNPs) exhibit remarkable mRNA delivery efficiency, yet their majority accumulate in the liver or spleen after injection. Tissue-specific mRNA delivery can be achieved through modulating LNP properties, such as tuning PEGylation or varying lipid components systematically. In this paper, a streamlined method is used for incorporating tumor-targeting peptides into the LNPs; the programmed death ligand 1 (PD-L1) binding peptides are conjugated to PEGylated lipids via a copper-free click reaction, and directly incorporated into the LNP composition (Pep LNPs). Notably, Pep LNPs display robust interaction with PD-L1 proteins, which leads to the uptake of LNPs into PD-L1 overexpressing cancer cells both in vitro and in vivo. To evaluate anticancer immunotherapy mediated by restoring tumor suppressor, mRNA encoding phosphatase and tensin homolog (PTEN) is delivered via Pep LNPs to PTEN-deficient triple-negative breast cancers (TNBCs). Pep LNPs loaded with PTEN mRNA specifically promotes autophagy-mediated immunogenic cell death in 4T1 tumors, resulting in effective anticancer immune responses. This study highlights the potential of tumor-targeted LNPs for mRNA-based cancer therapy.
Keywords: cancer immunotherapy; lipid nanoparticle; mRNA delivery; tumor‐targeted delivery.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

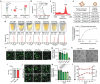

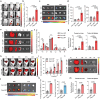
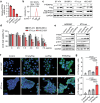



References
-
- Goyal F., Chattopadhyay A., Navik U., Jain A., Reddy P. H., Bhatti G. K., Bhatti J. S., Adv. Ther. 2024, 7, 2300255.
-
- a) Wang Q. T., Nie Y., Sun S. N., Lin T., Han R. J., Jiang J., Li Z., Li J. Q., Xiao Y. P., Fan Y. Y., Yuan X. H., Zhang H., Zhao B. B., Zeng M., Li S. Y., Liao H. X., Zhang J., He Y. W., Cancer Immunol. Immunother. 2020, 69, 1375; - PMC - PubMed
- b) Sahin U., Oehm P., Derhovanessian E., Jabulowsky R. A., Vormehr M., Gold M., Maurus D., Schwarck‐Kokarakis D., Kuhn A. N., Omokoko T., Kranz L. M., Diken M., Kreiter S., Haas H., Attig S., Rae R., Cuk K., Kemmer‐Brück A., Breitkreuz A., Tolliver C., Caspar J., Quinkhardt J., Hebich L., Stein M., Hohberger A., Vogler I., Liebig I., Renken S., Sikorski J., Leierer M., et al., Nature 2020, 585, 107; - PubMed
- c) Kreiter S., Vormehr M., van de Roemer N., Diken M., Lower M., Diekmann J., Boegel S., Schrors B., Vascotto F., Castle J. C., Tadmor A. D., Schoenberger S. P., Huber C., Tureci O., Sahin U., Nature 2015, 520, 692; - PMC - PubMed
- d) Balachandran V. P., Rojas L. A., Sethna Z., Soares K., Derhovanessian E., Mueller F., Yadav M., Basturk O., Gonen M., Wei A. C.‐c., D'Angelica M. I., Kingham T. P., Greenbaum B., Merghoub T., Jarnagin W. R., Drebin J. A., Sahin U., Tuereci O., Wolchok J. D., O'Reilly E. M., J. Clin. Oncol. 2022, 40, 2516;
- e) Kopetz S., Morris V. K., Alonso‐Orduña V., Garcia‐Alfonso P., Reboredo M., Montes A. F., Maurel J., Paez D., Reinacher‐Schick A. C., Höhler T., Carrasco J., Galligan B. M., Manning L., Preußner L., Tureci Ö., Sahin U., J. Clin. Oncol. 2022, 40, TPS3641.
-
- a) Xiao Y., Chen J., Zhou H., Zeng X., Ruan Z., Pu Z., Jiang X., Matsui A., Zhu L., Amoozgar Z., Chen D. S., Han X., Duda D. G., Shi J., Nat. Commun. 2022, 13, 758; - PMC - PubMed
- b) Lin Y. X., Wang Y., Ding J., Jiang A., Wang J., Yu M., Blake S., Liu S., Bieberich C. J., Farokhzad O. C., Mei L., Wang H., Shi J., Sci. Transl. Med. 2021, 13, eaba9772. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
