Preparation of human astrocytes with potent therapeutic functions from human pluripotent stem cells using ventral midbrain patterning
- PMID: 38521186
- PMCID: PMC11954835
- DOI: 10.1016/j.jare.2024.03.012
Preparation of human astrocytes with potent therapeutic functions from human pluripotent stem cells using ventral midbrain patterning
Abstract
Introduction: Astrocytes are glial-type cells that protect neurons from toxic insults and support neuronal functions and metabolism in a healthy brain. Leveraging these physiological functions, transplantation of astrocytes or their derivatives has emerged as a potential therapeutic approach for neurodegenerative disorders.
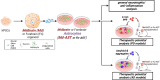
Methods: To substantiate the clinical application of astrocyte-based therapy, we aimed to prepare human astrocytes with potent therapeutic capacities from human pluripotent stem cells (hPSCs). To that end, we used ventral midbrain patterning during the differentiation of hPSCs into astrocytes, based on the roles of midbrain-specific factors in potentiating glial neurotrophic/anti-inflammatory activity. To assess the therapeutic effects of human midbrain-type astrocytes, we transplanted them into mouse models of Parkinson's disease (PD) and Alzheimer's disease (AD).
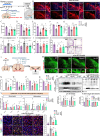
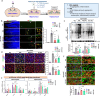
Results: Through a comprehensive series of in-vitro and in-vivo experiments, we were able to establish that the midbrain-type astrocytes exhibited the abilities to effectively combat oxidative stress, counter excitotoxic glutamate, and manage pathological protein aggregates. Our strategy for preparing midbrain-type astrocytes yielded promising results, demonstrating the strong therapeutic potential of these cells in various neurotoxic contexts. Particularly noteworthy is their efficacy in PD and AD-specific proteopathic conditions, in which the midbrain-type astrocytes outperformed forebrain-type astrocytes derived by the same organoid-based method.
Conclusion: The enhanced functions of the midbrain-type astrocytes extended to their ability to release signaling molecules that inhibited neuronal deterioration and senescence while steering microglial cells away from a pro-inflammatory state. This success was evident in both in-vitro studies using human cells and in-vivo experiments conducted in mouse models of PD and AD. In the end, our human midbrain-type astrocytes demonstrated remarkable effectiveness in alleviating neurodegeneration, neuroinflammation, and the pathologies associated with the accumulation of α-synuclein and Amyloid β proteins.
Keywords: Alzheimer’s disease; Amyloid β; Astrocyte; Parkinson’s disease; Transplantation; α-synuclein.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Neural stem cells derived from human midbrain organoids as a stable source for treating Parkinson's disease: Midbrain organoid-NSCs (Og-NSC) as a stable source for PD treatment.Prog Neurobiol. 2021 Sep;204:102086. doi: 10.1016/j.pneurobio.2021.102086. Epub 2021 May 28. Prog Neurobiol. 2021. PMID: 34052305
-
Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson's Disease.Stem Cell Reports. 2019 Feb 12;12(2):213-229. doi: 10.1016/j.stemcr.2018.12.011. Epub 2019 Jan 10. Stem Cell Reports. 2019. PMID: 30639209 Free PMC article.
-
Therapeutic functions of astrocytes to treat α-synuclein pathology in Parkinson's disease.Proc Natl Acad Sci U S A. 2022 Jul 19;119(29):e2110746119. doi: 10.1073/pnas.2110746119. Epub 2022 Jul 15. Proc Natl Acad Sci U S A. 2022. PMID: 35858361 Free PMC article.
-
Relationship Between Obesity, Alzheimer's Disease, and Parkinson's Disease: an Astrocentric View.Mol Neurobiol. 2017 Nov;54(9):7096-7115. doi: 10.1007/s12035-016-0193-8. Epub 2016 Oct 28. Mol Neurobiol. 2017. PMID: 27796748 Review.
-
Insights into the Molecular Mechanisms of Alzheimer's and Parkinson's Diseases with Molecular Simulations: Understanding the Roles of Artificial and Pathological Missense Mutations in Intrinsically Disordered Proteins Related to Pathology.Int J Mol Sci. 2018 Jan 24;19(2):336. doi: 10.3390/ijms19020336. Int J Mol Sci. 2018. PMID: 29364151 Free PMC article. Review.
Cited by
-
Human Pluripotent Stem Cell-Based Therapies for Parkinson's Disease: Challenges and Potential Solutions.Yonsei Med J. 2025 Jul;66(7):395-404. doi: 10.3349/ymj.2024.0447. Yonsei Med J. 2025. PMID: 40551588 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

