Tailored anticoagulant treatment after a first venous thromboembolism: protocol of the Leiden Thrombosis Recurrence Risk Prevention (L-TRRiP) study - cohort-based randomised controlled trial
- PMID: 38521524
- PMCID: PMC10961563
- DOI: 10.1136/bmjopen-2023-078676
Tailored anticoagulant treatment after a first venous thromboembolism: protocol of the Leiden Thrombosis Recurrence Risk Prevention (L-TRRiP) study - cohort-based randomised controlled trial
Abstract
Introduction: Patients with a first venous thromboembolism (VTE) are at risk of recurrence. Recurrent VTE (rVTE) can be prevented by extended anticoagulant therapy, but this comes at the cost of an increased risk of bleeding. It is still uncertain whether patients with an intermediate recurrence risk or with a high recurrence and high bleeding risk will benefit from extended anticoagulant treatment, and whether a strategy where anticoagulant duration is tailored on the predicted risks of rVTE and bleeding can improve outcomes. The aim of the Leiden Thrombosis Recurrence Risk Prevention (L-TRRiP) study is to evaluate the outcomes of tailored duration of long-term anticoagulant treatment based on individualised assessment of rVTE and major bleeding risks.
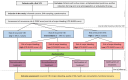
Methods and analysis: The L-TRRiP study is a multicentre, open-label, cohort-based, randomised controlled trial, including patients with a first VTE. We classify the risk of rVTE and major bleeding using the L-TRRiP and VTE-BLEED scores, respectively. After 3 months of anticoagulant therapy, patients with a low rVTE risk will discontinue anticoagulant treatment, patients with a high rVTE and low bleeding risk will continue anticoagulant treatment, whereas all other patients will be randomised to continue or discontinue anticoagulant treatment. All patients will be followed up for at least 2 years. Inclusion will continue until the randomised group consists of 608 patients; we estimate to include 1600 patients in total. The primary outcome is the combined incidence of rVTE and major bleeding in the randomised group after 2 years of follow-up. Secondary outcomes include the incidence of rVTE and major bleeding, functional outcomes, quality of life and cost-effectiveness in all patients.
Ethics and dissemination: The protocol was approved by the Medical Research Ethics Committee Leiden-Den Haag-Delft. Results are expected in 2028 and will be disseminated through peer-reviewed journals and during (inter)national conferences.
Trial registration number: NCT06087952.
Keywords: Anticoagulation; Clinical trials; EPIDEMIOLOGIC STUDIES; Thromboembolism.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MC has received financial support for research from Bayer, CSL Behring, Roche, Novo Nordisk and UniQure and lees for lecturing or consultancy from Alexion, Bayer, CSL Behring, Daiichi Sankyo, Sobi and Viatris, all unrelated to the present work and paid to his institution. NvE has received a lecture fee from Bristol Myers Squibb, which was unrelated to this work and paid to his institution. JL reports grants or contracts from BMS-Pfizer, Viatris, AstraZeneca and Synapse, all unrelated to this work and paid to her institution. KM reports speaker fees from Alexion, Bayer and CSL Behring, participation in trial steering committees for Bayer and AstraZeneca, consulting fees from Uniqure, participation in data monitoring and endpoint adjudication committee for Octapharma. All payments are made to her institution. SM reports grants and personal fees from Daiichi-Sankyo, Bayer, Pfizer and Boehringer-Ingelheim, personal fees from Portola/Alexion, AbbVie, Pfizer/Bristol-Meyers Squibb, Norgine, Viatris and Sanofi, all paid to her institution and outside the submitted work. MVH reports grants from Dutch Heart Foundation, Netherlands Organisation for Health Research and Development, Bayer Health Care, Pfizer-BMS Leo Pharma Boehringer-Ingelheim, all outside this work. FAK reports grants or contracts from Bayer, BMS, BSCI, MSD, Leo Pharma, Actelion, Farm-X, The Netherlands Organisation for Health Research and Development, the Dutch Thrombosis Association, The Dutch Heart Foundation and the Horizon Europe Program, all unrelated to this work and paid to his institution. All others report no conflicts of interest related to this project.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical