A unified model for regulating lipoprotein lipase activity
- PMID: 38521668
- PMCID: PMC11663433
- DOI: 10.1016/j.tem.2024.02.016
A unified model for regulating lipoprotein lipase activity
Abstract
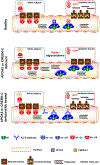
The regulation of triglyceride (TG) tissue distribution, storage, and utilization, a fundamental process of energy homeostasis, critically depends on lipoprotein lipase (LPL). We review the intricate mechanisms by which LPL activity is regulated by angiopoietin-like proteins (ANGPTL3, 4, 8), apolipoproteins (APOA5, APOC3, APOC2), and the cAMP-responsive element-binding protein H (CREBH). ANGPTL8 functions as a molecular switch, through complex formation, activating ANGPTL3 while deactivating ANGPTL4 in their LPL inhibition. The ANGPTL3-4-8 model integrates the roles of the aforementioned proteins in TG partitioning between white adipose tissue (WAT) and oxidative tissues (heart and skeletal muscles) during the feed/fast cycle. This model offers a unified perspective on LPL regulation, providing insights into TG metabolism, metabolic diseases, and therapeutics.
Keywords: ANGPTL3; ANGPTL4; ANGPTL8; APOA5; APOC2; APOC3; CREBH; lipoprotein lipase.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflicts of interest.
Figures



References
-
- Speakman JR (2013) Evolutionary perspectives on the obesity epidemic: adaptive, maladaptive, and neutral viewpoints. Annu. Rev. Nutr 33, 289–317 - PubMed
-
- Shelness GS and Sellers JA (2001) Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol 12, 151–157 - PubMed
-
- Hahn PF (1943) Abolishment of alimentary lipemia following injection of heparin. Science 98, 19–20 - PubMed
-
- Korn ED (1954) Properties of clearing factor obtained from rat heart acetone powder. Science 120, 399–400 - PubMed
-
- Wang H and Eckel RH (2009) Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab 297, E271–E288 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

