Targeted therapy for immune mediated skin diseases. What should a dermatologist know?
- PMID: 38521706
- PMCID: PMC11221168
- DOI: 10.1016/j.abd.2023.10.002
Targeted therapy for immune mediated skin diseases. What should a dermatologist know?
Abstract
Background: Molecularly targeted therapies, such as monoclonal antibodies (mAbs) and Janus Kinase inhibitors (JAKis), have emerged as essential tools in the treatment of dermatological diseases. These therapies modulate the immune system through specific signaling pathways, providing effective alternatives to traditional systemic immunosuppressive agents. This review aims to provide an updated summary of targeted immune therapies for inflammatory skin diseases, considering their pathophysiology, efficacy, dosage, and safety profiles.
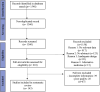
Methods: The review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. A systematic search was conducted on PubMed over the past 10 years, focusing on randomized clinical trials, case reports, and case series related to targeted immune therapies in dermatology. Eligibility criteria were applied, and data were extracted from each study, including citation data, study design, and results.
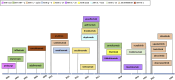
Results: We identified 1360 non-duplicate articles with the initial search strategy. Title and abstract review excluded 1150, while a full-text review excluded an additional 50 articles. The review included 143 studies published between 2012 and 2022, highlighting 39 drugs currently under investigation or in use for managing inflammatory skin diseases.
Study limitations: The heterogeneity of summarized information limits this review. Some recommendations originated from data from clinical trials, while others relied on retrospective analyses and small case series. Recommendations will likely be updated as new results emerge.
Conclusion: Targeted therapies have revolutionized the treatment of chronic skin diseases, offering new options for patients unresponsive to standard treatments. Paradoxical reactions are rarely observed. Further studies are needed to fully understand the mechanisms and nature of these therapies. Overall, targeted immune therapies in dermatology represent a promising development, significantly improving the quality of life for patients with chronic inflammatory skin diseases.
Keywords: Atopic dermatitis; Biologics; Immune modulators; Inflammatory skin diseases; Jak inhibitors; Psoriasis; Targeted therapy.
Copyright © 2024. Published by Elsevier España, S.L.U.
Figures
References
-
- Buss N.A.P.S., Henderson S.J., McFarlane M., Shenton J.M., de Haan L. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol. 2012;12:615–622. - PubMed
-
- Duvall M.R., Fiorini R.N. Different approaches for obtaining antibodies from human B cells. Curr Drug Discov Technol. 2014;11:41–47. - PubMed
-
- Bolognia J., Schaffer J., Cerroni L. 4th edition. Elsevier; Edinburgh: 2017. Dermatology; p. 2880.
-
- Yeung Y.T., Aziz F., Guerrero-Castilla A., Arguelles S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr Pharm Des. 2018;24:1449–1484. - PubMed
-
- Yamauchi P.S. 1st ed. Springer International Publishing: Imprint: Springer; Cham: 2018. Biologic and systemic agents in dermatology; p. 1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous