Astrocytes in selective vulnerability to neurodegenerative disease
- PMID: 38521710
- PMCID: PMC11006581
- DOI: 10.1016/j.tins.2024.02.008
Astrocytes in selective vulnerability to neurodegenerative disease
Abstract
Selective vulnerability of specific brain regions and cell populations is a hallmark of neurodegenerative disorders. Mechanisms of selective vulnerability involve neuronal heterogeneity, functional specializations, and differential sensitivities to stressors and pathogenic factors. In this review we discuss the growing body of literature suggesting that, like neurons, astrocytes are heterogeneous and specialized, respond to and integrate diverse inputs, and induce selective effects on brain function. In disease, astrocytes undergo specific, context-dependent changes that promote different pathogenic trajectories and functional outcomes. We propose that astrocytes contribute to selective vulnerability through maladaptive transitions to context-divergent phenotypes that impair specific brain regions and functions. Further studies on the multifaceted roles of astrocytes in disease may provide new therapeutic approaches to enhance resilience against neurodegenerative disorders.
Keywords: dementia; glia; heterogeneity; neuropathology; proteinopathy; resilience.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests in relation to this work.
Figures
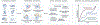


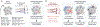
Similar articles
-
Reduced expression of Pss gene in Drosophila cortex glia causes dopaminergic cell death.J Parkinsons Dis. 2025 Aug;15(5):957-969. doi: 10.1177/1877718X251349407. Epub 2025 Jun 16. J Parkinsons Dis. 2025. PMID: 40518954
-
Lesion-remote astrocytes govern microglia-mediated white matter repair.bioRxiv [Preprint]. 2024 Mar 17:2024.03.15.585251. doi: 10.1101/2024.03.15.585251. bioRxiv. 2024. PMID: 38558977 Free PMC article. Preprint.
-
hiPSC-neurons recapitulate the subtype-specific cell intrinsic nature of susceptibility to neurodegenerative disease-relevant aggregation.Acta Neuropathol Commun. 2025 May 19;13(1):108. doi: 10.1186/s40478-025-02000-4. Acta Neuropathol Commun. 2025. PMID: 40390134 Free PMC article.
-
Δ133p53α-mediated inhibition of astrocyte senescence and neurotoxicity as a possible therapeutic approach for neurodegenerative diseases.Neuroscience. 2025 Aug 6;580:54-61. doi: 10.1016/j.neuroscience.2025.06.031. Epub 2025 Jun 14. Neuroscience. 2025. PMID: 40523602 Free PMC article. Review.
-
Neurotrophomodulatory effect of TNF-α through NF-κB in rat cortical astrocytes.Cytotechnology. 2025 Feb;77(1):37. doi: 10.1007/s10616-024-00698-z. Epub 2025 Jan 5. Cytotechnology. 2025. PMID: 39776978 Review.
Cited by
-
PD-linked LRRK2 G2019S mutation impairs astrocyte morphology and synapse maintenance via ERM hyperphosphorylation.bioRxiv [Preprint]. 2025 Apr 25:2023.04.09.536178. doi: 10.1101/2023.04.09.536178. bioRxiv. 2025. PMID: 39253496 Free PMC article. Preprint.
-
Defective Astrocyte Maturation Drives Cerebellar Neuroinflammation and Degeneration.FASEB J. 2025 Jul 31;39(14):e70824. doi: 10.1096/fj.202501225RR. FASEB J. 2025. PMID: 40641255 Free PMC article.
-
Neuroimmune signaling mediates astrocytic nucleocytoplasmic disruptions and stress granule formation associated with TDP-43 pathology.Neurobiol Dis. 2025 Jul;211:106939. doi: 10.1016/j.nbd.2025.106939. Epub 2025 May 9. Neurobiol Dis. 2025. PMID: 40339618 Free PMC article.
-
Heterogeneous brain region-specific responses to astrocytic mitochondrial DNA damage in mice.Sci Rep. 2024 Aug 10;14(1):18586. doi: 10.1038/s41598-024-69499-w. Sci Rep. 2024. PMID: 39127716 Free PMC article.
-
Brain Metabolism in Health and Neurodegeneration: The Interplay Among Neurons and Astrocytes.Cells. 2024 Oct 17;13(20):1714. doi: 10.3390/cells13201714. Cells. 2024. PMID: 39451233 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

