Plasma p-tau212 antemortem diagnostic performance and prediction of autopsy verification of Alzheimer's disease neuropathology
- PMID: 38521766
- PMCID: PMC10960791
- DOI: 10.1038/s41467-024-46876-7
Plasma p-tau212 antemortem diagnostic performance and prediction of autopsy verification of Alzheimer's disease neuropathology
Abstract
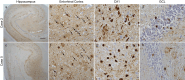
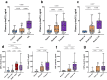
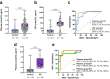
Blood phosphorylated tau (p-tau) biomarkers, including p-tau217, show high associations with Alzheimer's disease (AD) neuropathologic change and clinical stage. Certain plasma p-tau217 assays recognize tau forms phosphorylated additionally at threonine-212, but the contribution of p-tau212 alone to AD is unknown. We developed a blood-based immunoassay that is specific to p-tau212 without cross-reactivity to p-tau217. Here, we examined the diagnostic utility of plasma p-tau212. In five cohorts (n = 388 participants), plasma p-tau212 showed high performances for AD diagnosis and for the detection of both amyloid and tau pathology, including at autopsy as well as in memory clinic populations. The diagnostic accuracy and fold changes of plasma p-tau212 were similar to those for p-tau217 but higher than p-tau181 and p-tau231. Immunofluorescent staining of brain tissue slices showed prominent p-tau212 reactivity in neurofibrillary tangles that co-localized with p-tau217 and p-tau202/205. These findings support plasma p-tau212 as a peripherally accessible biomarker of AD pathophysiology.
© 2024. The Author(s).
Conflict of interest statement
M.T. and P.H. are employees of Bioventix Plc. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche. K.B. has served as a consultant or at advisory boards for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers. H.Z. and K.B. are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. NJA has given lectures in symposia sponsored by Lilly, BioArctic, and Quanterix. The other authors declare no competing interests.
Figures




Update of
-
Plasma p-tau212: antemortem diagnostic performance and prediction of autopsy verification of Alzheimer's disease neuropathology.medRxiv [Preprint]. 2023 Dec 11:2023.12.11.23299806. doi: 10.1101/2023.12.11.23299806. medRxiv. 2023. Update in: Nat Commun. 2024 Mar 23;15(1):2615. doi: 10.1038/s41467-024-46876-7. PMID: 38168323 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R01 AG075336/AG/NIA NIH HHS/United States
- R01 AG083874/AG/NIA NIH HHS/United States
- R01 AG072641/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- R01 AG068398/AG/NIA NIH HHS/United States
- R21 AG078538/AG/NIA NIH HHS/United States
- R01 MH108509/MH/NIMH NIH HHS/United States
- RF1 AG025516/AG/NIA NIH HHS/United States
- P30 AG066468/AG/NIA NIH HHS/United States
- R01 AG073267/AG/NIA NIH HHS/United States
- P01 AG025204/AG/NIA NIH HHS/United States
- #AARF-21-850325/ALZ/Alzheimer's Association/United States
- R01 MH121619/MH/NIMH NIH HHS/United States
- P30 AG066507/AG/NIA NIH HHS/United States
- R01 AG078796/AG/NIA NIH HHS/United States
- R37 AG023651/AG/NIA NIH HHS/United States
- R21 AG080705/AG/NIA NIH HHS/United States
- U24 AG082930/AG/NIA NIH HHS/United States
- R01 AG025516/AG/NIA NIH HHS/United States
- RF1 AG052525/AG/NIA NIH HHS/United States
- R01 AG053952/AG/NIA NIH HHS/United States

