A molecular extraction process for vanadium based on tandem selective complexation and precipitation
- PMID: 38521785
- PMCID: PMC10960790
- DOI: 10.1038/s41467-024-46958-6
A molecular extraction process for vanadium based on tandem selective complexation and precipitation
Abstract
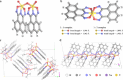
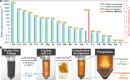
Recycling vanadium from alternative sources is essential due to its expanding demand, depletion in natural sources, and environmental issues with terrestrial mining. Here, we present a complexation-precipitation method to selectively recover pentavalent vanadium ions, V(V), from complex metal ion mixtures, using an acid-stable metal binding agent, the cyclic imidedioxime, naphthalimidedioxime (H2CIDIII). H2CIDIII showed high extraction capacity and fast binding towards V(V) with crystal structures showing a 1:1 M:L dimer, [V2(O)3(C12H6N3O2)2]2-, 1, and 1:2 M:L non-oxido, [V(C12H6N3O2)2] ̶ complex, 2. Complexation selectivity studies showed only 1 and 2 were anionic, allowing facile separation of the V(V) complexes by pH-controlled precipitation, removing the need for solid support. The tandem complexation-precipitation technique achieved high recovery selectivity for V(V) with a selectivity coefficient above 3 × 105 from synthetic mixed metal solutions and real oil sand tailings. Zebrafish toxicity assay confirmed the non-toxicity of 1 and 2, highlighting H2CIDIII's potential for practical and large-scale V(V) recovery.
© 2024. The Author(s).
Conflict of interest statement
We have filed a provisional patent on this approach with O.A.O. and G.K.H.S. as inventors. The remaining authors declare no competing interests.
Figures




Similar articles
-
Enhanced selective separation of vanadium(V) and chromium(VI) using the CeO2 nanorod containing oxygen vacancies.Environ Sci Pollut Res Int. 2023 Jun;30(27):70731-70741. doi: 10.1007/s11356-023-27415-1. Epub 2023 May 8. Environ Sci Pollut Res Int. 2023. PMID: 37155091
-
On-column complexation and simultaneous separation of vanadium(IV) and vanadium(V) by capillary electrophoresis with direct UV detection.Anal Bioanal Chem. 2002 Oct;374(3):520-5. doi: 10.1007/s00216-002-1456-y. Epub 2002 Sep 14. Anal Bioanal Chem. 2002. PMID: 12373403
-
1H and 51V NMR investigations of the molecular nature of implant-derived vanadium ions in osteoarthritic knee-joint synovial fluid.Clin Chim Acta. 2007 May 1;380(1-2):89-99. doi: 10.1016/j.cca.2007.01.015. Epub 2007 Jan 24. Clin Chim Acta. 2007. PMID: 17346687
-
Kinetics of complexation of V(v), U(vi), and Fe(iii) with glutaroimide-dioxime: studies by stopped-flow and conventional absorption spectroscopy.Dalton Trans. 2017 Aug 22;46(33):11084-11096. doi: 10.1039/c7dt01597f. Dalton Trans. 2017. PMID: 28787059
-
Separation of vanadium, tungsten and molybdenum from spent SCR catalysts solution by solvent extraction with primary amine N1923.Waste Manag. 2022 Aug 1;150:301-309. doi: 10.1016/j.wasman.2022.07.015. Epub 2022 Jul 22. Waste Manag. 2022. PMID: 35878529 Review.
Cited by
-
Robust biomimetic MOF featuring a negative pocket for precise recognition of uranyl, enabling ultrahigh U/V selectivity and rapid uranium extraction from seawater.Chem Sci. 2025 Jun 20;16(30):13749-13759. doi: 10.1039/d5sc02966j. eCollection 2025 Jul 30. Chem Sci. 2025. PMID: 40607058 Free PMC article.
References
-
- Yuan R, et al. A critical review on extraction and refining of vanadium metal. Int. J. Refract. Met. Hard Mater. 2021;101:105696. doi: 10.1016/j.ijrmhm.2021.105696. - DOI
-
- Chan W, Dogan O, King P. Thermodynamic assessment of V-rare earth systems. J. Phase Equilib. Diffus. 2010;31:425–432. doi: 10.1007/s11669-010-9779-4. - DOI
-
- Moskalyk RR, Alfantazi AM. Processing of vanadium: a review. Miner. Eng. 2003;16:793–805. doi: 10.1016/S0892-6875(03)00213-9. - DOI
-
- Stas J, Dahdouh A, Al-chayah O. Recovery of vanadium, nickel and molybdenum from fly ash of heavy oil-fired electrical power station. Period. Polytech. Chem. Eng. 2007;51:67–70. doi: 10.3311/pp.ch.2007-2.11. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

