Voluntary exercise preserves visual function and reduces inflammatory response in an adult mouse model of autosomal dominant retinitis pigmentosa
- PMID: 38521799
- PMCID: PMC10960803
- DOI: 10.1038/s41598-024-57027-9
Voluntary exercise preserves visual function and reduces inflammatory response in an adult mouse model of autosomal dominant retinitis pigmentosa
Abstract
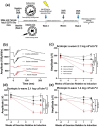
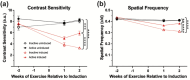
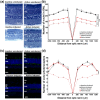
Whole-body physical exercise has been shown to promote retinal structure and function preservation in animal models of retinal degeneration. It is currently unknown how exercise modulates retinal inflammatory responses. In this study, we investigated cytokine alterations associated with retinal neuroprotection induced by voluntary running wheel exercise in a retinal degeneration mouse model of class B1 autosomal dominant retinitis pigmentosa, I307N Rho. I307N Rho mice undergo rod photoreceptor degeneration when exposed to bright light (induced). Our data show, active induced mice exhibited significant preservation of retinal and visual function compared to inactive induced mice after 4 weeks of exercise. Retinal cytokine expression revealed significant reductions of proinflammatory chemokines, keratinocyte-derived chemokine (KC) and interferon gamma inducible protein-10 (IP-10) expression in active groups compared to inactive groups. Through immunofluorescence, we found KC and IP-10 labeling localized to retinal vasculature marker, collagen IV. These data show that whole-body exercise lowers specific retinal cytokine expression associated with retinal vasculature. Future studies should determine whether suppression of inflammatory responses is requisite for exercise-induced retinal protection.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Wheel running exercise protects against retinal degeneration in the I307N rhodopsin mouse model of inducible autosomal dominant retinitis pigmentosa.Mol Vis. 2019 Aug 21;25:462-476. eCollection 2019. Mol Vis. 2019. PMID: 31523123 Free PMC article.
-
Protein kinase G inhibition preserves photoreceptor viability and function in a new mouse model for autosomal dominant retinitis pigmentosa.Cell Death Dis. 2025 Jul 30;16(1):575. doi: 10.1038/s41419-025-07901-9. Cell Death Dis. 2025. PMID: 40738887 Free PMC article.
-
Rhodopsin mislocalization drives ciliary dysregulation in a novel autosomal dominant retinitis pigmentosa knock-in mouse model.FASEB J. 2024 Apr 30;38(8):e23606. doi: 10.1096/fj.202302260RR. FASEB J. 2024. PMID: 38648465 Free PMC article.
-
Vitamin A and fish oils for retinitis pigmentosa.Cochrane Database Syst Rev. 2013 Dec 19;2013(12):CD008428. doi: 10.1002/14651858.CD008428.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Jun 18;6(6):CD008428. doi: 10.1002/14651858.CD008428.pub3. PMID: 24357340 Free PMC article. Updated.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
Cited by
-
Bidirectional causality of physical exercise in retinal neuroprotection.Neural Regen Res. 2025 Dec 1;20(12):3400-3415. doi: 10.4103/NRR.NRR-D-24-00942. Epub 2024 Dec 16. Neural Regen Res. 2025. PMID: 39688575 Free PMC article.
-
Effects of treadmill exercise on retinal vascular morphology, function, and circulating immune factors in a mouse model of retinal degeneration.bioRxiv [Preprint]. 2025 Feb 12:2025.02.11.637694. doi: 10.1101/2025.02.11.637694. bioRxiv. 2025. Update in: Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):65. doi: 10.1167/iovs.66.11.65. PMID: 39990328 Free PMC article. Updated. Preprint.
-
Voluntary running partially prevents photoreceptor cell death in retinitis pigmentosa.Front Neurosci. 2025 Apr 25;19:1563607. doi: 10.3389/fnins.2025.1563607. eCollection 2025. Front Neurosci. 2025. PMID: 40352907 Free PMC article.
-
Forced exercise modulates retinal inflammatory response and regulates miRNA expression to promote retinal neuroprotection during degeneration.bioRxiv [Preprint]. 2025 Jul 21:2025.07.16.665187. doi: 10.1101/2025.07.16.665187. bioRxiv. 2025. PMID: 40777372 Free PMC article. Preprint.
-
Effects of Treadmill Exercise on Retinal Vascular Morphology, Function, and Circulating Immune Factors in a Mouse Model of Retinal Degeneration.Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):65. doi: 10.1167/iovs.66.11.65. Invest Ophthalmol Vis Sci. 2025. PMID: 40856647 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

