Inflammation-induced TRPV4 channels exacerbate blood-brain barrier dysfunction in multiple sclerosis
- PMID: 38521959
- PMCID: PMC10960997
- DOI: 10.1186/s12974-024-03069-9
Inflammation-induced TRPV4 channels exacerbate blood-brain barrier dysfunction in multiple sclerosis
Abstract
Background: Blood-brain barrier (BBB) dysfunction and immune cell migration into the central nervous system (CNS) are pathogenic drivers of multiple sclerosis (MS). Ways to reinstate BBB function and subsequently limit neuroinflammation present promising strategies to restrict disease progression. However, to date, the molecular players directing BBB impairment in MS remain poorly understood. One suggested candidate to impact BBB function is the transient receptor potential vanilloid-type 4 ion channel (TRPV4), but its specific role in MS pathogenesis remains unclear. Here, we investigated the role of TRPV4 in BBB dysfunction in MS.
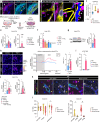
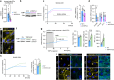
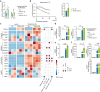
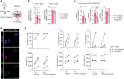
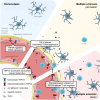
Main text: In human post-mortem MS brain tissue, we observed a region-specific increase in endothelial TRPV4 expression around mixed active/inactive lesions, which coincided with perivascular microglia enrichment in the same area. Using in vitro models, we identified that microglia-derived tumor necrosis factor-α (TNFα) induced brain endothelial TRPV4 expression. Also, we found that TRPV4 levels influenced brain endothelial barrier formation via expression of the brain endothelial tight junction molecule claudin-5. In contrast, during an inflammatory insult, TRPV4 promoted a pathological endothelial molecular signature, as evidenced by enhanced expression of inflammatory mediators and cell adhesion molecules. Moreover, TRPV4 activity mediated T cell extravasation across the brain endothelium.
Conclusion: Collectively, our findings suggest a novel role for endothelial TRPV4 in MS, in which enhanced expression contributes to MS pathogenesis by driving BBB dysfunction and immune cell migration.
Keywords: Blood–brain barrier; Multiple sclerosis; T cells; TNFα; TRPV4; Vessel-associated microglia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the absence of any potential conflict of interest.
Figures

References
-
- Izquierdo G, Hauw JJ, Lyon-Caen O, Marteau R, Escourolle R, Buge A, et al. Clinical analysis of 70 neuropathologic cases of multiple sclerosis. Rev Neurol (Paris) 1985;141(8–9):546–552. - PubMed
-
- Van Waesberghe JHTM, Kamphorst W, De Groot CJA, Van Walderveen MAA, Castelijns JA, Ravid R, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46(5):747–754. doi: 10.1002/1531-8249(199911)46:5<747::AID-ANA10>3.0.CO;2-4. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 813294/European Union´s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant (ENTRAIN)
- 813294/European Union´s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant (ENTRAIN)
- 813294/European Union´s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant (ENTRAIN)
- 813294/European Union´s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant (ENTRAIN)
- 91719305/Dutch Research Council, NWO, Vidi grant
- 91719305/Dutch Research Council, NWO, Vidi grant
- 91719305/Dutch Research Council, NWO, Vidi grant
- 18-1023MS/Stichting MS Research
- 20-1106MS/Stichting MS Research
- 20-1106MS/Stichting MS Research
- 18-1023MS/Stichting MS Research
- 20-1106MS/Stichting MS Research
- 81X3100216/Deutsches Zentrum für Herz-Kreislaufforschung
- SFB-TR84 : subprojects A02 & C09, SFB-1449 subproject B01, SFB 1470 subproject A04, KU1218/9-1, KU1218/11-1, and KU1218/12-1/Deutsche Forschungsgemeinschaft
- PROVID (01KI20160A) and SYMPATH (01ZX1906A)/Bundesministerium für Bildung und Forschung
- HA2016-02-02/Hersenstichting
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

