A role for TGFβ signaling in Gli1+ tendon and enthesis cells
- PMID: 38522021
- PMCID: PMC10962263
- DOI: 10.1096/fj.202301452R
A role for TGFβ signaling in Gli1+ tendon and enthesis cells
Abstract
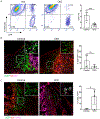
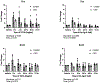
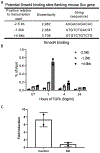
The development of musculoskeletal tissues such as tendon, enthesis, and bone relies on proliferation and differentiation of mesenchymal progenitor cells. Gli1+ cells have been described as putative stem cells in several tissues and are presumed to play critical roles in tissue formation and maintenance. For example, the enthesis, a fibrocartilage tissue that connects tendon to bone, is mineralized postnatally by a pool of Gli1+ progenitor cells. These cells are regulated by hedgehog signaling, but it is unclear if TGFβ signaling, necessary for tenogenesis, also plays a role in their behavior. To examine the role of TGFβ signaling in Gli1+ cell function, the receptor for TGFβ, TbR2, was deleted in Gli1-lineage cells in mice at P5. Decreased TGFβ signaling in these cells led to defects in tendon enthesis formation by P56, including defective bone morphometry underlying the enthesis and decreased mechanical properties. Immunohistochemical staining of these Gli1+ cells showed that loss of TGFβ signaling reduced proliferation and increased apoptosis. In vitro experiments using Gli1+ cells isolated from mouse tail tendons demonstrated that TGFβ controls cell proliferation and differentiation through canonical and non-canonical pathways and that TGFβ directly controls the tendon transcription factor scleraxis by binding to its distant enhancer. These results have implications in the development of treatments for tendon and enthesis pathologies.
Keywords: Gli1; TGFβ; TbR2; development; enthesis.
© 2024 Federation of American Societies for Experimental Biology.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Figures



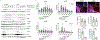
Similar articles
-
Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis.Dev Biol. 2015 Sep 1;405(1):96-107. doi: 10.1016/j.ydbio.2015.06.020. Epub 2015 Jun 30. Dev Biol. 2015. PMID: 26141957 Free PMC article.
-
Enthesis regeneration: a role for Gli1+ progenitor cells.Development. 2017 Apr 1;144(7):1159-1164. doi: 10.1242/dev.139303. Epub 2017 Feb 20. Development. 2017. PMID: 28219952 Free PMC article.
-
Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment.Development. 2015 Jan 1;142(1):196-206. doi: 10.1242/dev.112714. Development. 2015. PMID: 25516975 Free PMC article.
-
Hedgehog signaling underlying tendon and enthesis development and pathology.Matrix Biol. 2022 Jan;105:87-103. doi: 10.1016/j.matbio.2021.12.001. Epub 2021 Dec 24. Matrix Biol. 2022. PMID: 34954379 Free PMC article. Review.
-
Key Roles of Gli1 and Ihh Signaling in Craniofacial Development.Stem Cells Dev. 2024 Jun;33(11-12):251-261. doi: 10.1089/scd.2024.0036. Epub 2024 May 3. Stem Cells Dev. 2024. PMID: 38623785 Review.
References
-
- Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, and Basler KJN (2018) GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. 558, 449–453 - PubMed
-
- Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, and Gratwohl S. J. C. s. c. (2016) Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. 19, 628–642 - PMC - PubMed
-
- Men Y, Wang Y, Yi Y, Jing D, Luo W, Shen B, Stenberg W, Chai Y, Ge W-P, and Feng JQJDC (2020) Gli1+ periodontium stem cells are regulated by osteocytes and occlusal force. 54, 639–654. e636 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

