A bacterial toxin co-opts caspase-3 to disable active gasdermin D and limit macrophage pyroptosis
- PMID: 38522070
- PMCID: PMC11095105
- DOI: 10.1016/j.celrep.2024.114004
A bacterial toxin co-opts caspase-3 to disable active gasdermin D and limit macrophage pyroptosis
Abstract
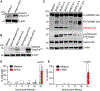
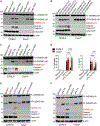
During infections, host cells are exposed to pathogen-associated molecular patterns (PAMPs) and virulence factors that stimulate multiple signaling pathways that interact additively, synergistically, or antagonistically. The net effect of such higher-order interactions is a vital determinant of the outcome of host-pathogen interactions. Here, we demonstrate one such complex interplay between bacterial exotoxin- and PAMP-induced innate immune pathways. We show that two caspases activated during enterohemorrhagic Escherichia coli (EHEC) infection by lipopolysaccharide (LPS) and Shiga toxin (Stx) interact in a functionally antagonistic manner; cytosolic LPS-activated caspase-11 cleaves full-length gasdermin D (GSDMD), generating an active pore-forming N-terminal fragment (NT-GSDMD); subsequently, caspase-3 activated by EHEC Stx cleaves the caspase-11-generated NT-GSDMD to render it nonfunctional, thereby inhibiting pyroptosis and interleukin-1β maturation. Bacteria typically subvert inflammasomes by targeting upstream components such as NLR sensors or full-length GSDMD but not active NT-GSDMD. Thus, our findings uncover a distinct immune evasion strategy where a bacterial toxin disables active NT-GSDMD by co-opting caspase-3.
Keywords: CP: Immunology; CP: Microbiology; EHEC; Shiga toxin; caspase-11; caspase-3; enterohemorrhagic E. coli; gasdermin D; noncanonical inflammasome; pyroptosis.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

