Aziridination via Nitrogen-Atom Transfer to Olefins from Photoexcited Azoxy-Triazenes
- PMID: 38522088
- PMCID: PMC11009954
- DOI: 10.1021/jacs.3c14713
Aziridination via Nitrogen-Atom Transfer to Olefins from Photoexcited Azoxy-Triazenes
Abstract
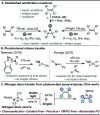
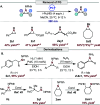
Herein, we report that readily accessible azoxy-triazenes can serve as nitrogen atom sources under visible light excitation for the phthalimido-protected aziridination of alkenes. This approach eliminates the need for external oxidants, precious transition metals, and photocatalysts, marking a departure from conventional methods. The versatility of this transformation extends to the selective aziridination of both activated and unactivated multisubstituted alkenes of varying electronic profiles. Notably, this process avoids the formation of competing C-H insertion products. The described protocol is operationally simple, scalable, and adaptable to photoflow conditions. Mechanistic studies support the idea that the photofragmentation of azoxy-triazenes results in the generation of a free singlet nitrene. Furthermore, a mild photoredox-catalyzed N-N cleavage of the protecting group to furnish the free aziridines is reported. Our findings contribute to the advancement of sustainable and practical methodologies for the synthesis of nitrogen-containing compounds, showcasing the potential for broader applications in synthetic chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Direct and Stereospecific Synthesis of N-H and N-Alkyl Aziridines from Unactivated Olefins Using Hydroxylamine-O-Sulfonic Acids.Angew Chem Int Ed Engl. 2017 Aug 7;56(33):9886-9890. doi: 10.1002/anie.201705530. Epub 2017 Jul 12. Angew Chem Int Ed Engl. 2017. PMID: 28614619 Free PMC article.
-
Organic Dye-Sensitized Nitrene Generation: Intermolecular Aziridination of Unactivated Alkenes.J Org Chem. 2024 Mar 1;89(5):3251-3258. doi: 10.1021/acs.joc.3c02709. Epub 2024 Feb 15. J Org Chem. 2024. PMID: 38358354 Free PMC article.
-
Chiral Cpx Rhodium(III)-Catalyzed Enantioselective Aziridination of Unactivated Terminal Alkenes.Angew Chem Int Ed Engl. 2024 Mar 18;63(12):e202400502. doi: 10.1002/anie.202400502. Epub 2024 Feb 13. Angew Chem Int Ed Engl. 2024. PMID: 38279683
-
Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer.Acc Chem Res. 2021 Mar 2;54(5):1209-1225. doi: 10.1021/acs.accounts.0c00591. Epub 2021 Jan 25. Acc Chem Res. 2021. PMID: 33491448 Free PMC article. Review.
-
Dehydrogenative Pd and Ni Catalysis for Total Synthesis.Acc Chem Res. 2021 Mar 2;54(5):1118-1130. doi: 10.1021/acs.accounts.0c00787. Epub 2021 Feb 16. Acc Chem Res. 2021. PMID: 33592147 Free PMC article. Review.
Cited by
-
Aziridine Group Transfer via Transient N-Aziridinyl Radicals.J Am Chem Soc. 2024 Nov 13;146(45):30796-30801. doi: 10.1021/jacs.4c14169. Epub 2024 Nov 4. J Am Chem Soc. 2024. PMID: 39497240 Free PMC article.
-
Accessing Azetidines through Magnesium-Mediated Nitrogen Group Transfer from Iminoiodinane to Donor-Acceptor Cyclopropanes.Angew Chem Int Ed Engl. 2025 Feb 17;64(8):e202420485. doi: 10.1002/anie.202420485. Epub 2025 Jan 21. Angew Chem Int Ed Engl. 2025. PMID: 39776232 Free PMC article.
-
Is Phenylnitrene a Missing Link in the Formation of Polycyclic Aromatic Nitrogen Heterocycles?Angew Chem Int Ed Engl. 2025 Jul 28;64(31):e202503940. doi: 10.1002/anie.202503940. Epub 2025 Jun 17. Angew Chem Int Ed Engl. 2025. PMID: 40326970 Free PMC article.
-
Nitrene-mediated aminative N-N-N coupling: facile access to triazene 1-oxides.Chem Sci. 2025 Mar 10;16(15):6458-6467. doi: 10.1039/d5sc00064e. eCollection 2025 Apr 9. Chem Sci. 2025. PMID: 40103730 Free PMC article.
-
C=C Dissociative Imination of Styrenes by a Photogenerated Metallonitrene.JACS Au. 2024 Sep 3;4(9):3421-3426. doi: 10.1021/jacsau.4c00571. eCollection 2024 Sep 23. JACS Au. 2024. PMID: 39328761 Free PMC article.
References
-
- Singh G. S.Advances in Synthesis and Chemistry of Aziridines. In Advances in Heterocyclic Chemistry ;Elsevier, 2019; Vol. 129, pp 245–33510.1016/bs.aihch.2018.12.003. - DOI
-
- Sabir S.; Kumar G.; Verma V. P.; Jat J. L. Aziridine Ring Opening: An Overview of Sustainable Methods. ChemistrySelect 2018, 3, 3702–3711. 10.1002/slct.201800170. - DOI
-
- Padwa A.Aziridines and Azirines: Monocyclic. In Comprehensive Heterocyclic Chemistry III ;Elsevier, 2008; Vol. 1, pp 1–104.
Grants and funding
LinkOut - more resources
Full Text Sources

