SARS-CoV-2 infection modifies the transcriptome of the megakaryocytes in the bone marrow
- PMID: 38522092
- PMCID: PMC11176959
- DOI: 10.1182/bloodadvances.2023012367
SARS-CoV-2 infection modifies the transcriptome of the megakaryocytes in the bone marrow
Abstract
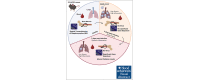
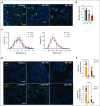
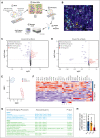
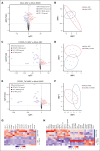
Megakaryocytes (MKs), integral to platelet production, predominantly reside in the bone marrow (BM) and undergo regulated fragmentation within sinusoid vessels to release platelets into the bloodstream. Inflammatory states and infections influence MK transcription, potentially affecting platelet functionality. Notably, COVID-19 has been associated with altered platelet transcriptomes. In this study, we investigated the hypothesis that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection could affect the transcriptome of BM MKs. Using spatial transcriptomics to discriminate subpopulations of MKs based on proximity to BM sinusoids, we identified ∼19 000 genes in MKs. Machine learning techniques revealed that the transcriptome of healthy murine BM MKs exhibited minimal differences based on proximity to sinusoid vessels. Furthermore, at peak SARS-CoV-2 viremia, when the disease primarily affected the lungs, MKs were not significantly different from those from healthy mice. Conversely, a significant divergence in the MK transcriptome was observed during systemic inflammation, although SARS-CoV-2 RNA was never detected in the BM, and it was no longer detectable in the lungs. Under these conditions, the MK transcriptional landscape was enriched in pathways associated with histone modifications, MK differentiation, NETosis, and autoimmunity, which could not be explained by cell proximity to sinusoid vessels. Notably, the type I interferon signature and calprotectin (S100A8/A9) were not induced in MKs under any condition. However, inflammatory cytokines induced in the blood and lungs of COVID-19 mice were different from those found in the BM, suggesting a discriminating impact of inflammation on this specific subset of cells. Collectively, our data indicate that a new population of BM MKs may emerge through COVID-19-related pathogenesis.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

Similar articles
-
Single-cell analysis of ploidy and the transcriptome reveals functional and spatial divergency in murine megakaryopoiesis.Blood. 2021 Oct 7;138(14):1211-1224. doi: 10.1182/blood.2021010697. Blood. 2021. PMID: 34115843 Free PMC article.
-
Comparative transcriptome analyses reveal genes associated with SARS-CoV-2 infection of human lung epithelial cells.Sci Rep. 2021 Aug 10;11(1):16212. doi: 10.1038/s41598-021-95733-w. Sci Rep. 2021. PMID: 34376762 Free PMC article.
-
CXCL12-Abundant Reticular (CAR) Cells Direct Megakaryocyte Protrusions across the Bone Marrow Sinusoid Wall.Cells. 2021 Mar 24;10(4):722. doi: 10.3390/cells10040722. Cells. 2021. PMID: 33804965 Free PMC article.
-
Occurrence and role of lung megakaryocytes in infection and inflammation.Front Immunol. 2022 Nov 29;13:1029223. doi: 10.3389/fimmu.2022.1029223. eCollection 2022. Front Immunol. 2022. PMID: 36524131 Free PMC article. Review.
-
The bone marrow niche from the inside out: how megakaryocytes are shaped by and shape hematopoiesis.Blood. 2022 Jan 27;139(4):483-491. doi: 10.1182/blood.2021012827. Blood. 2022. PMID: 34587234 Free PMC article. Review.
Cited by
-
Megakaryocyte in sepsis: the trinity of coagulation, inflammation and immunity.Crit Care. 2024 Dec 31;28(1):442. doi: 10.1186/s13054-024-05221-6. Crit Care. 2024. PMID: 39741325 Free PMC article. Review.
-
Immune and Inflammatory Properties of Megakaryocytes.Cells. 2025 Jul 10;14(14):1053. doi: 10.3390/cells14141053. Cells. 2025. PMID: 40710306 Free PMC article. Review.
References
-
- Gong Y, Zhao M, Yang W, et al. Megakaryocyte-derived excessive transforming growth factor beta1 inhibits proliferation of normal hematopoietic stem cells in acute myeloid leukemia. Exp Hematol. 2018;60:40–46.e2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

