Nanoparticle targeting of neutrophil glycolysis prevents lung ischemia-reperfusion injury
- PMID: 38522826
- PMCID: PMC11305958
- DOI: 10.1016/j.ajt.2024.03.028
Nanoparticle targeting of neutrophil glycolysis prevents lung ischemia-reperfusion injury
Abstract
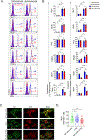
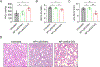
Neutrophils exacerbate pulmonary ischemia-reperfusion injury (IRI) resulting in poor short and long-term outcomes for lung transplant recipients. Glycolysis powers neutrophil activation, but it remains unclear if neutrophil-specific targeting of this pathway will inhibit IRI. Lipid nanoparticles containing the glycolysis flux inhibitor 2-deoxyglucose (2-DG) were conjugated to neutrophil-specific Ly6G antibodies (NP-Ly6G[2-DG]). Intravenously administered NP-Ly6G(2-DG) to mice exhibited high specificity for circulating neutrophils. NP-Ly6G(2-DG)-treated neutrophils were unable to adapt to hypoglycemic conditions of the lung airspace environment as evident by the loss of demand-induced glycolysis, reductions in glycogen and ATP content, and an increased vulnerability to apoptosis. NP-Ly6G(2-DG) treatment inhibited pulmonary IRI following hilar occlusion and orthotopic lung transplantation. IRI protection was associated with less airspace neutrophil extracellular trap generation, reduced intragraft neutrophilia, and enhanced alveolar macrophage efferocytotic clearance of neutrophils. Collectively, our data show that pharmacologically targeting glycolysis in neutrophils inhibits their activation and survival leading to reduced pulmonary IRI.
Keywords: acute lung injury and immunosuppression; efferocytosis; glycolysis; immunometabolism; ischemia-reperfusion injury; lung transplant; nanoparticles; neutrophils; primary graft dysfunction.
Copyright © 2024 American Society of Transplantation & American Society of Transplant Surgeons. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures
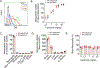




References
-
- Gelman AE, Fisher AJ, Huang HJ, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part III: Mechanisms: A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. Oct 2017;36(10):1114–1120. doi: 10.1016/j.healun.2017.07.014 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

