Harnessing innate immune pathways for therapeutic advancement in cancer
- PMID: 38523155
- PMCID: PMC10961329
- DOI: 10.1038/s41392-024-01765-9
Harnessing innate immune pathways for therapeutic advancement in cancer
Abstract
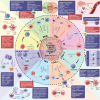
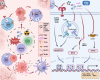
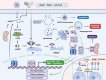
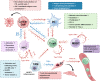
The innate immune pathway is receiving increasing attention in cancer therapy. This pathway is ubiquitous across various cell types, not only in innate immune cells but also in adaptive immune cells, tumor cells, and stromal cells. Agonists targeting the innate immune pathway have shown profound changes in the tumor microenvironment (TME) and improved tumor prognosis in preclinical studies. However, to date, the clinical success of drugs targeting the innate immune pathway remains limited. Interestingly, recent studies have shown that activation of the innate immune pathway can paradoxically promote tumor progression. The uncertainty surrounding the therapeutic effectiveness of targeted drugs for the innate immune pathway is a critical issue that needs immediate investigation. In this review, we observe that the role of the innate immune pathway demonstrates heterogeneity, linked to the tumor development stage, pathway status, and specific cell types. We propose that within the TME, the innate immune pathway exhibits multidimensional diversity. This diversity is fundamentally rooted in cellular heterogeneity and is manifested as a variety of signaling networks. The pro-tumor effect of innate immune pathway activation essentially reflects the suppression of classical pathways and the activation of potential pro-tumor alternative pathways. Refining our understanding of the tumor's innate immune pathway network and employing appropriate targeting strategies can enhance our ability to harness the anti-tumor potential of the innate immune pathway and ultimately bridge the gap from preclinical to clinical application.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

