RhoA downregulation in the murine intestinal epithelium results in chronic Wnt activation and increased tumorigenesis
- PMID: 38523777
- PMCID: PMC10959657
- DOI: 10.1016/j.isci.2024.109400
RhoA downregulation in the murine intestinal epithelium results in chronic Wnt activation and increased tumorigenesis
Abstract
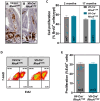
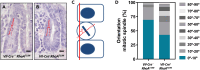
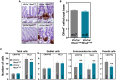
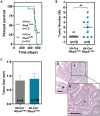
Rho GTPases are molecular switches regulating multiple cellular processes. To investigate the role of RhoA in normal intestinal physiology, we used a conditional mouse model overexpressing a dominant negative RhoA mutant (RhoAT19N) in the intestinal epithelium. Although RhoA inhibition did not cause an overt phenotype, increased levels of nuclear β-catenin were observed in the small intestinal epithelium of RhoAT19N mice, and the overexpression of multiple Wnt target genes revealed a chronic activation of Wnt signaling. Elevated Wnt signaling in RhoAT19N mice and intestinal organoids did not affect the proliferation of intestinal epithelial cells but significantly interfered with their differentiation. Importantly, 17-month-old RhoAT19N mice showed a significant increase in the number of spontaneous intestinal tumors. Altogether, our results indicate that RhoA regulates the differentiation of intestinal epithelial cells and inhibits tumor initiation, likely through the control of Wnt signaling, a key regulator of proliferation and differentiation in the intestine.
Keywords: Cancer; Cell biology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

