Distinct functional neutrophil phenotypes in sepsis patients correlate with disease severity
- PMID: 38524125
- PMCID: PMC10957777
- DOI: 10.3389/fimmu.2024.1341752
Distinct functional neutrophil phenotypes in sepsis patients correlate with disease severity
Abstract
Purpose: Sepsis is a clinical syndrome defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Sepsis is a highly heterogeneous syndrome with distinct phenotypes that impact immune function and response to infection. To develop targeted therapeutics, immunophenotyping is needed to identify distinct functional phenotypes of immune cells. In this study, we utilized our Organ-on-Chip assay to categorize sepsis patients into distinct phenotypes using patient data, neutrophil functional analysis, and proteomics.
Methods: Following informed consent, neutrophils and plasma were isolated from sepsis patients in the Temple University Hospital ICU (n=45) and healthy control donors (n=7). Human lung microvascular endothelial cells (HLMVEC) were cultured in the Organ-on-Chip and treated with buffer or cytomix ((TNF/IL-1β/IFNγ). Neutrophil adhesion and migration across HLMVEC in the Organ-on-Chip were used to categorize functional neutrophil phenotypes. Quantitative label-free global proteomics was performed on neutrophils to identify differentially expressed proteins. Plasma levels of sepsis biomarkers and neutrophil extracellular traps (NETs) were determined by ELISA.
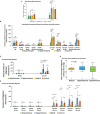
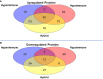
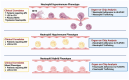
Results: We identified three functional phenotypes in critically ill ICU sepsis patients based on ex vivo neutrophil adhesion and migration patterns. The phenotypes were classified as: Hyperimmune characterized by enhanced neutrophil adhesion and migration, Hypoimmune that was unresponsive to stimulation, and Hybrid with increased adhesion but blunted migration. These functional phenotypes were associated with distinct proteomic signatures and differentiated sepsis patients by important clinical parameters related to disease severity. The Hyperimmune group demonstrated higher oxygen requirements, increased mechanical ventilation, and longer ICU length of stay compared to the Hypoimmune and Hybrid groups. Patients with the Hyperimmune neutrophil phenotype had significantly increased circulating neutrophils and elevated plasma levels NETs.
Conclusion: Neutrophils and NETs play a critical role in vascular barrier dysfunction in sepsis and elevated NETs may be a key biomarker identifying the Hyperimmune group. Our results establish significant associations between specific neutrophil functional phenotypes and disease severity and identify important functional parameters in sepsis pathophysiology that may provide a new approach to classify sepsis patients for specific therapeutic interventions.
Keywords: Organ-on-Chip; neutrophil extracellular traps; neutrophil heterogeneity; proteomics; sepsis.
Copyright © 2024 Yang, Langston, Prosniak, Pettigrew, Zhao, Perez, Edelmann, Mansoor, Merali, Merali, Marchetti, Prabhakarpandian, Kiani and Kilpatrick.
Conflict of interest statement
Author BP was employed by the company CFD Research Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

