Optimal Once-Daily Busulfan Administration in Pediatric Patients: A Simulation-Based Investigation of Intravenous Infusion Times
- PMID: 38524879
- PMCID: PMC10961087
- DOI: 10.2147/DDDT.S451970
Optimal Once-Daily Busulfan Administration in Pediatric Patients: A Simulation-Based Investigation of Intravenous Infusion Times
Abstract
Purpose: Pediatric patients receiving hematopoietic stem cell transplantation undergo regular administration of intravenous busulfan as a conditioning regimen. Once-daily regimen of busulfan has been proposed as a more convenient alternative to the traditional regimen, but it may increase the risk of toxicity such as veno-occlusive disease (VOD). The study aims to evaluate the pharmacokinetics (PKs) of once-daily regimens and investigate appropriate intravenous infusion times to reduce the risk of toxicity.
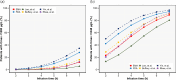
Patients and methods: Once-daily busulfan dosing regimens for pediatric patient were reviewed and selected including EMA- and FDA-based once-daily dosing regimens. We generated busulfan PK data of virtual pediatric patients using a previously developed population PK model. PK profiles and proportion of patients achieving the referenced maximum concentration (Cmax) and exposure to busulfan were used to evaluate the appropriateness of both infusion time and dosing regimens.
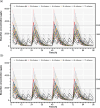
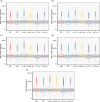
Results: Predicted PK profiles and exposure of busulfan showed relatively similar distributions for all once-daily dosing regimens. Most patients exceeded the referenced Cmax possibly associated with a high risk of VOD with all once-daily regimens when applied with 3 hours of infusion.
Conclusion: While intravenous infusion of once-daily busulfan is typically administered over 3 hours, our findings emphasize the necessity of considering sufficient infusion times to ensure safe drug utilization and prevent toxicity, which will aid in optimal busulfan use in pediatric oncology.
Keywords: busulfan; infusion times; once-daily dosing regimen; pediatrics; population pharmacokinetics.
© 2024 Kim et al.
Conflict of interest statement
The authors have no conflicts of interest to disclose in this work.
Figures
Similar articles
-
Pediatric patients undergoing hematopoietic stem cell transplantation can greatly benefit from a novel once-daily intravenous busulfan dosing nomogram.Am J Hematol. 2017 Jul;92(7):607-613. doi: 10.1002/ajh.24734. Epub 2017 May 30. Am J Hematol. 2017. PMID: 28370238
-
Test Dose Pharmacokinetics in Pediatric Patients Receiving Once-Daily IV Busulfan Conditioning for Hematopoietic Stem Cell Transplant: A Reliable Approach?J Clin Pharmacol. 2018 Mar;58(3):332-339. doi: 10.1002/jcph.1049. Epub 2017 Dec 14. J Clin Pharmacol. 2018. PMID: 29238995 Free PMC article.
-
Association Between the Magnitude of Intravenous Busulfan Exposure and Development of Hepatic Veno-Occlusive Disease in Children and Young Adults Undergoing Myeloablative Allogeneic Hematopoietic Cell Transplantation.Transplant Cell Ther. 2022 Apr;28(4):196-202. doi: 10.1016/j.jtct.2022.01.013. Epub 2022 Jan 19. Transplant Cell Ther. 2022. PMID: 35065280
-
Evaluation of safety and pharmacokinetics of administering intravenous busulfan in a twice-daily or daily schedule to patients with advanced hematologic malignant disease undergoing stem cell transplantation.Biol Blood Marrow Transplant. 2002;8(9):486-92. doi: 10.1053/bbmt.2002.v8.pm12374453. Biol Blood Marrow Transplant. 2002. PMID: 12374453 Clinical Trial.
-
Therapeutic drug monitoring of busulfan in transplantation.Curr Pharm Des. 2008;14(20):1936-49. doi: 10.2174/138161208785061382. Curr Pharm Des. 2008. PMID: 18691105 Review.
Cited by
-
Comparison of busulfan pharmacokinetics between four-times-daily and once-daily administration in pediatric patients: a preliminary prospective observational trial.Int J Hematol. 2025 Feb;121(2):244-251. doi: 10.1007/s12185-024-03891-0. Epub 2024 Dec 3. Int J Hematol. 2025. PMID: 39625679
-
Ferroptotic Pathway Activation in Spermatogonia: A Novel Mechanism of Busulfan-Induced Testicular Injury.Biology (Basel). 2025 May 23;14(6):594. doi: 10.3390/biology14060594. Biology (Basel). 2025. PMID: 40563846 Free PMC article.
-
Once-Daily Versus Four-Times-Daily Intravenous Busulfan with Therapeutic Drug Monitoring as Conditioning for Hematopoietic Cell Transplantation in Children.Pharmaceutics. 2025 Aug 21;17(8):1081. doi: 10.3390/pharmaceutics17081081. Pharmaceutics. 2025. PMID: 40871100 Free PMC article.
References
-
- Bartelink IH, Lalmohamed A, van Reij EM, et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol. 2016;3(11):e526–e536. doi:10.1016/S2352-3026(16)30114-4 - DOI - PMC - PubMed
-
- Lee JW, Kang HJ, Lee SH, et al. Highly variable pharmacokinetics of once-daily intravenous busulfan when combined with fludarabine in pediatric patients: Phase I clinical study for determination of optimal once-daily busulfan dose using pharmacokinetic modeling. Biol Blood Marrow Transplant. 2012;18(6):944–950. doi:10.1016/j.bbmt.2011.11.025 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

