Optimal Once-Daily Busulfan Administration in Pediatric Patients: A Simulation-Based Investigation of Intravenous Infusion Times
- PMID: 38524879
- PMCID: PMC10961087
- DOI: 10.2147/DDDT.S451970
Optimal Once-Daily Busulfan Administration in Pediatric Patients: A Simulation-Based Investigation of Intravenous Infusion Times
Abstract
Purpose: Pediatric patients receiving hematopoietic stem cell transplantation undergo regular administration of intravenous busulfan as a conditioning regimen. Once-daily regimen of busulfan has been proposed as a more convenient alternative to the traditional regimen, but it may increase the risk of toxicity such as veno-occlusive disease (VOD). The study aims to evaluate the pharmacokinetics (PKs) of once-daily regimens and investigate appropriate intravenous infusion times to reduce the risk of toxicity.
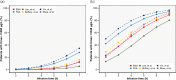
Patients and methods: Once-daily busulfan dosing regimens for pediatric patient were reviewed and selected including EMA- and FDA-based once-daily dosing regimens. We generated busulfan PK data of virtual pediatric patients using a previously developed population PK model. PK profiles and proportion of patients achieving the referenced maximum concentration (Cmax) and exposure to busulfan were used to evaluate the appropriateness of both infusion time and dosing regimens.
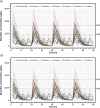
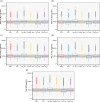
Results: Predicted PK profiles and exposure of busulfan showed relatively similar distributions for all once-daily dosing regimens. Most patients exceeded the referenced Cmax possibly associated with a high risk of VOD with all once-daily regimens when applied with 3 hours of infusion.
Conclusion: While intravenous infusion of once-daily busulfan is typically administered over 3 hours, our findings emphasize the necessity of considering sufficient infusion times to ensure safe drug utilization and prevent toxicity, which will aid in optimal busulfan use in pediatric oncology.
Keywords: busulfan; infusion times; once-daily dosing regimen; pediatrics; population pharmacokinetics.
© 2024 Kim et al.
Conflict of interest statement
The authors have no conflicts of interest to disclose in this work.
Figures
References
-
- Bartelink IH, Lalmohamed A, van Reij EM, et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol. 2016;3(11):e526–e536. doi: 10.1016/S2352-3026(16)30114-4 - DOI - PMC - PubMed
-
- Lee JW, Kang HJ, Lee SH, et al. Highly variable pharmacokinetics of once-daily intravenous busulfan when combined with fludarabine in pediatric patients: Phase I clinical study for determination of optimal once-daily busulfan dose using pharmacokinetic modeling. Biol Blood Marrow Transplant. 2012;18(6):944–950. doi: 10.1016/j.bbmt.2011.11.025 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

