Emerging Strategies to Overcome Current CAR-T Therapy Dilemmas - Exosomes Derived from CAR-T Cells
- PMID: 38525009
- PMCID: PMC10959326
- DOI: 10.2147/IJN.S445101
Emerging Strategies to Overcome Current CAR-T Therapy Dilemmas - Exosomes Derived from CAR-T Cells
Abstract
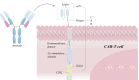
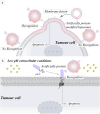
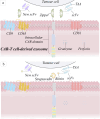
Adoptive T cells immunotherapy, specifically chimeric antigen receptor T cells (CAR-T), has shown promising therapeutic efficacy in the treatment of hematologic malignancies. As extensive research on CAR-T therapies has been conducted, various challenges have emerged that significantly hampered their clinical application, including tumor recurrence, CAR-T cell exhaustion, and cytokine release syndrome (CRS). To overcome the hurdles of CAR-T therapy in clinical treatment, cell-free emerging therapies based on exosomes derived from CAR-T cells have been developed as an effective and promising alternative approach. In this review, we present CAR-T cell-based therapies for the treatment of tumors, including the features and benefits of CAR-T therapies, the limitations that exist in this field, and the measures taken to overcome them. Furthermore, we discuss the notable benefits of utilizing exosomes released from CAR-T cells in tumor treatment and anticipate potential issues in clinical trials. Lastly, drawing from previous research on exosomes from CAR-T cells and the characteristics of exosomes, we propose strategies to overcome these restrictions. Additionally, the review discusses the plight in large-scale preparation of exosome and provides potential solutions for future clinical applications.
Keywords: CAR-T cells; exosome; immune escape; tumor.
© 2024 Hu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Innate and Innate-Like Cells: The Future of Chimeric Antigen Receptor (CAR) Cell Therapy.Trends Pharmacol Sci. 2021 Jan;42(1):45-59. doi: 10.1016/j.tips.2020.11.004. Epub 2020 Nov 26. Trends Pharmacol Sci. 2021. PMID: 33250273 Review.
-
CAR-T Cell Therapy: Challenges and Optimization.Crit Rev Immunol. 2021;41(1):77-87. doi: 10.1615/CritRevImmunol.2021037253. Crit Rev Immunol. 2021. PMID: 33822526 Review.
-
Nanotechnology and immunoengineering: How nanotechnology can boost CAR-T therapy.Acta Biomater. 2020 Jun;109:21-36. doi: 10.1016/j.actbio.2020.04.015. Epub 2020 Apr 13. Acta Biomater. 2020. PMID: 32294554 Review.
-
CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy.J Exp Clin Cancer Res. 2022 Mar 31;41(1):119. doi: 10.1186/s13046-022-02327-z. J Exp Clin Cancer Res. 2022. PMID: 35361234 Free PMC article. Review.
-
Chimeric Antigen Receptor T-Cell Therapy: Current Perspective on T Cell-Intrinsic, T Cell-Extrinsic, and Therapeutic Limitations.Cancer J. 2023 Jan-Feb 01;29(1):28-33. doi: 10.1097/PPO.0000000000000636. Cancer J. 2023. PMID: 36693155 Review.
Cited by
-
Nanoplatelets modified with RVG for targeted delivery of miR-375 and temozolomide to enhance gliomas therapy.J Nanobiotechnology. 2024 Oct 15;22(1):623. doi: 10.1186/s12951-024-02895-6. J Nanobiotechnology. 2024. PMID: 39402578 Free PMC article.
-
Composition, functions, and applications of exosomal membrane proteins.Front Immunol. 2024 Aug 1;15:1408415. doi: 10.3389/fimmu.2024.1408415. eCollection 2024. Front Immunol. 2024. PMID: 39148736 Free PMC article. Review.
-
Empowering brain tumor management: chimeric antigen receptor macrophage therapy.Theranostics. 2024 Sep 3;14(14):5725-5742. doi: 10.7150/thno.98290. eCollection 2024. Theranostics. 2024. PMID: 39310093 Free PMC article. Review.
-
CAR-T lymphocyte-based cell therapies; mechanistic substantiation, applications and biosafety enhancement with suicide genes: new opportunities to melt side effects.Front Immunol. 2024 Jul 18;15:1333150. doi: 10.3389/fimmu.2024.1333150. eCollection 2024. Front Immunol. 2024. PMID: 39091493 Free PMC article. Review.
-
Harnessing Tumor Cell-Derived Exosomes for Immune Rejection Management in Corneal Transplantation.Adv Sci (Weinh). 2025 Jan;12(2):e2409207. doi: 10.1002/advs.202409207. Epub 2024 Nov 14. Adv Sci (Weinh). 2025. PMID: 39540242 Free PMC article.
References
-
- Naeem M, Hazafa A, Bano N, et al. Explorations of CRISPR/Cas9 for improving the long-term efficacy of universal CAR-T cells in tumor immunotherapy. Life Sci. 2023;2023:316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

