Nano-Drug Delivery Systems Targeting CAFs: A Promising Treatment for Pancreatic Cancer
- PMID: 38525013
- PMCID: PMC10959015
- DOI: 10.2147/IJN.S451151
Nano-Drug Delivery Systems Targeting CAFs: A Promising Treatment for Pancreatic Cancer
Abstract
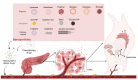
Currently, pancreatic cancer (PC) is one of the most lethal malignant tumors. PC is typically diagnosed at a late stage, exhibits a poor response to conventional treatment, and has a bleak prognosis. Unfortunately, PC's survival rate has not significantly improved since the 1960s. Cancer-associated fibroblasts (CAFs) are a key component of the pancreatic tumor microenvironment (TME). They play a vital role in maintaining the extracellular matrix and facilitating the intricate communication between cancer cells and infiltrated immune cells. Exploring therapeutic approaches targeting CAFs may reverse the current landscape of PC therapy. In recent years, nano-drug delivery systems have evolved rapidly and have been able to accurately target and precisely release drugs with little or no toxicity to the whole body. In this review, we will comprehensively discuss the origin, heterogeneity, potential targets, and recent advances in the nano-drug delivery system of CAFs in PC. We will also propose a novel integrated treatment regimen that utilizes a nano-drug delivery system to target CAFs in PC, combined with radiotherapy and immunotherapy. Additionally, we will address the challenges that this regimen currently faces.
Keywords: cancer-associated fibroblasts; nano-drug delivery system; nanoparticle; pancreatic cancer; tumor microenvironment.
© 2024 Wang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Drug Delivery System Targeting Cancer-Associated Fibroblast for Improving Immunotherapy.Int J Nanomedicine. 2025 Jan 11;20:483-503. doi: 10.2147/IJN.S500591. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39816375 Free PMC article. Review.
-
Nanoparticle-based delivery systems modulate the tumor microenvironment in pancreatic cancer for enhanced therapy.J Nanobiotechnology. 2021 Nov 22;19(1):384. doi: 10.1186/s12951-021-01134-6. J Nanobiotechnology. 2021. PMID: 34809634 Free PMC article. Review.
-
Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts.Adv Drug Deliv Rev. 2021 May;172:37-51. doi: 10.1016/j.addr.2021.02.012. Epub 2021 Mar 8. Adv Drug Deliv Rev. 2021. PMID: 33705881 Review.
-
Emerging Nano Drug Delivery Systems Targeting Cancer-Associated Fibroblasts for Improved Antitumor Effect and Tumor Drug Penetration.Mol Pharm. 2020 Apr 6;17(4):1028-1048. doi: 10.1021/acs.molpharmaceut.0c00014. Epub 2020 Mar 16. Mol Pharm. 2020. PMID: 32150417 Review.
-
Cancer-associated fibroblasts: A key target to snatch victory from defeat in therapy resistance associated with the pancreatic cancer stroma.Cancer Lett. 2023 Jul 28;567:216279. doi: 10.1016/j.canlet.2023.216279. Epub 2023 Jun 17. Cancer Lett. 2023. PMID: 37336287 Review.
Cited by
-
Drug Delivery System Targeting Cancer-Associated Fibroblast for Improving Immunotherapy.Int J Nanomedicine. 2025 Jan 11;20:483-503. doi: 10.2147/IJN.S500591. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39816375 Free PMC article. Review.
-
For and against tumor microenvironment: Nanoparticle-based strategies for active cancer therapy.Mater Today Bio. 2025 Mar 1;31:101626. doi: 10.1016/j.mtbio.2025.101626. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40124335 Free PMC article. Review.
-
Advances in nanotechnology for targeting cancer-associated fibroblasts: A review of multi-strategy drug delivery and preclinical insights.APL Bioeng. 2025 Mar 13;9(1):011502. doi: 10.1063/5.0244706. eCollection 2025 Mar. APL Bioeng. 2025. PMID: 40094065 Free PMC article. Review.
-
Enhancing the Efficacy of Active Pharmaceutical Ingredients in Medicinal Plants through Nanoformulations: A Promising Field.Nanomaterials (Basel). 2024 Oct 3;14(19):1598. doi: 10.3390/nano14191598. Nanomaterials (Basel). 2024. PMID: 39404324 Free PMC article. Review.
-
Harnessing the tumor microenvironment: targeted cancer therapies through modulation of epithelial-mesenchymal transition.J Hematol Oncol. 2025 Jan 13;18(1):6. doi: 10.1186/s13045-024-01634-6. J Hematol Oncol. 2025. PMID: 39806516 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

