Visible-Light-Driven Mentha spicata L.-Mediated Ag-Doped Bi2Zr2O7 Nanocomposite for Enhanced Degradation of Organic Pollutants, Electrochemical Sensing, and Antibacterial Applications
- PMID: 38525021
- PMCID: PMC10958660
- DOI: 10.1021/acsenvironau.3c00057
Visible-Light-Driven Mentha spicata L.-Mediated Ag-Doped Bi2Zr2O7 Nanocomposite for Enhanced Degradation of Organic Pollutants, Electrochemical Sensing, and Antibacterial Applications
Abstract
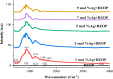
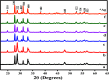
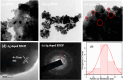
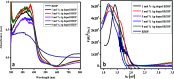
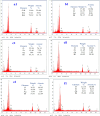
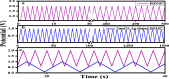
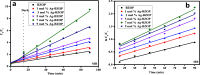
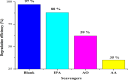
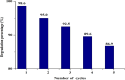
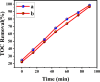
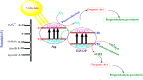
Novel visible-light-driven Ag (X)-doped Bi2Zr2O7 (BZO) nanocomposites in pudina (P) extract (Mentha spicata L.), X-1, 3, 5, 7, and 9 mol %, were synthesized by the one-pot greener solution combustion method. The as-synthesized nanocomposite materials were characterized by using various spectral [X-ray diffraction (XRD), Fourier transform infrared, UV-visible, UV- diffuse reflectance spectra, X-ray photoelectron spectroscopy], electrochemical (cyclic voltammetry, electrochemical impedance spectroscopy), and analytical (scanning electron microscopy-energy-dispersive X-ray spectroscopy, transmission electron microscopy, Brunauer-Emmett-Teller) techniques. The average particle size of the nanocomposite material was found to be between 14.8 and 39.2 nm by XRD. The well-characterized Ag-doped BZOP nanocomposite materials exhibited enhanced photocatalytic degradation activity toward hazardous dyes such as methylene blue (MB) and rose bengal (RB) under visible light irradiation ranges between 400 and 800 nm due to their low energy band gap. As a result, 7 mol % of Ag-doped BZOP nanocomposite material exhibited excellent photodegradation activity against MB (D.E. = 98.7%) and RB (D.E. = 99.3%) as compared to other Ag-doped BZOP nanocomposite materials and pure BZOP nanocomposite, respectively, due to enhanced semiconducting and optical behaviors, high binding energy, and mechanical and thermal stabilities. The Ag-doped BZOP nanocomposite material-based electrochemical sensor showed good sensing ability toward the determination of lead nitrate and dextrose with the lowest limit of detection (LOD) of 18 μM and 12 μM, respectively. Furthermore, as a result of the initial antibacterial screening study, the Ag-doped BZOP nanocomposite material was found to be more effective against Gram-negative bacteria (Escherichia coli) as compared to Gram-positive (Staphylococcus aureus) bacteria. The scavenger study reveals that radicals such as O2•- and •OH are responsible for MB and RB mineralization. TOC removal percentages were found to be 96.8 and 98.5% for MB and RB dyes, and experimental data reveal that the Ag-doped BZOP enhances the radical (O2•- and •OH) formation and MB and RB degradation under visible-light irradiation.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
Green-engineered synthesis of Bi2Zr2O7 NPs: excellent performance on electrochemical sensor and sunlight-driven photocatalytic studies.Environ Sci Pollut Res Int. 2023 Jan 5. doi: 10.1007/s11356-022-24760-5. Online ahead of print. Environ Sci Pollut Res Int. 2023. PMID: 36602731
-
Silver-Doped BaSrTiO₃ Nanocomposite: Synthesis, Characterization, Antibacterial and Photocatalytic Activities.J Nanosci Nanotechnol. 2021 Oct 1;21(10):5131-5142. doi: 10.1166/jnn.2021.19340. J Nanosci Nanotechnol. 2021. PMID: 33875098
-
Fabrication of Reduced Graphene Oxide Decorated with Nonmetal-Doped Nanotitania: An Efficient Visible Light-Driven Photocatalyst and Sterilizing Agent for Microbial Cells.ACS Omega. 2025 Feb 10;10(6):5296-5311. doi: 10.1021/acsomega.4c05015. eCollection 2025 Feb 18. ACS Omega. 2025. PMID: 39989826 Free PMC article.
-
Coupling ZnO with CuO for efficient organic pollutant removal.Environ Sci Pollut Res Int. 2023 Jun;30(28):71984-72008. doi: 10.1007/s11356-022-24139-6. Epub 2022 Nov 22. Environ Sci Pollut Res Int. 2023. PMID: 36414902
-
A facile synthesis process of GCN/ZnO-Cu nanocomposite and the evaluation of the performance for the photocatalytic degradation of organic pollutants and the disinfection of wastewater under visible light.Chemosphere. 2023 Dec;344:140287. doi: 10.1016/j.chemosphere.2023.140287. Epub 2023 Oct 10. Chemosphere. 2023. PMID: 37820879 Review.
Cited by
-
Green synthesis of trimetallic CuO/Ag/ZnO nanocomposite using Ziziphus spina-christi plant extract: characterization, statistically experimental designs, and antimicrobial assessment.Sci Rep. 2024 Aug 24;14(1):19718. doi: 10.1038/s41598-024-67579-5. Sci Rep. 2024. PMID: 39181914 Free PMC article.
-
A nano-powered green and chemically synthesized Au/MWCNT modified electrochemical sensor for methylene blue detection in river water.Nanoscale Adv. 2025 Aug 13. doi: 10.1039/d5na00396b. Online ahead of print. Nanoscale Adv. 2025. PMID: 40822880 Free PMC article.
References
-
- Liu X.; Huang L.; Wu X.; Wang Z.; Dong G.; Wang C.; Liu Y.; Wang L. Bi2Zr2O7 nanoparticles synthesized by soft-templated sol-gel methods for visible-light-driven catalytic degradation of tetracycline. Chemosphere 2018, 210, 424–432. 10.1016/j.chemosphere.2018.07.040. - DOI - PubMed
- Sabri M.; Habibi-Yangjeh A.; Rahim Pouran S.; Wang C. Titania-activated persulfate for environmental remediation: the-state-of-the-art. Catal. Rev. 2023, 65, 118–173. 10.1080/01614940.2021.1996776. - DOI
-
- Qu Z.; Jing Z.; Chen X.; Wang Z.; Ren H.; Huang L. Preparation and photocatalytic performance study of dual Z-scheme Bi2Zr2O7/g-C3N4/Ag3PO4 for removal of antibiotics by visible-light. J. Environ. Sci. 2023, 125, 349–361. 10.1016/j.jes.2022.01.010. - DOI - PubMed
- Feizpoor S.; Rahim Pouran S.; Habibi-Yangjeh A. Recent progress on photocatalytic evolution of hydrogen gas over TiO2-x-based emerging nanostructures. Mater. Sci. Semicond. Process. 2023, 162, 107444.10.1016/j.mssp.2023.107444. - DOI
- Habibi-Yangjeh A.; Pournemati K. A review on emerging homojunction photocatalysts with impressive performances for wastewater detoxification. Crit. Rev. Environ. Sci. Technol. 2023, 0, 1–31. 10.1080/10643389.2023.2239125. - DOI
-
- Sun X.; Gu M.; Yang J.; Ye G.; Xiao X.; Chen M.; Liu M.; Chen Z.; Huang H. The photocatalytic performances of Bi2MTaO7 (M = Ga, In) photocatalysts for environmental cleaning under visible-light. Inorg. Chem. Commun. 2022, 139, 109390.10.1016/j.inoche.2022.109390. - DOI
-
- Huang D.; Li J.; Zeng G.; Xue W.; Chen S.; Li Z.; Deng R.; Yang Y.; Cheng M. Facile construction of hierarchical flower-like Z-scheme AgBr/Bi2WO6 photocatalysts for effective removal of tetracycline: Degradation pathways and mechanism. J. Chem. Eng. 2019, 375, 121991.10.1016/j.cej.2019.121991. - DOI
-
- Maham M.; Nasrollahzadeh M.; Mohammad Sajadi S. Facile synthesis of Ag/ZrO2 nanocomposite as a recyclable catalyst for the treatment of environmental pollutants. Composites, Part B 2020, 185, 107783.10.1016/j.compositesb.2020.107783. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
