Which cues are sexy? The evolution of mate preference in sympatric species reveals the contrasted effect of adaptation and reproductive interference
- PMID: 38525034
- PMCID: PMC10959492
- DOI: 10.1093/evlett/qrad058
Which cues are sexy? The evolution of mate preference in sympatric species reveals the contrasted effect of adaptation and reproductive interference
Abstract
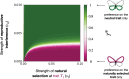
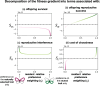
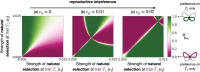
Mate preferences may target traits (a) enhancing offspring adaptation and (b) reducing heterospecific matings. Because similar selective pressures are acting on traits shared by different sympatric species, preference-enhancing offspring adaptation may increase heterospecific mating, in sharp contrast with the classical case of so-called "magic traits." Using a mathematical model, we study which and how many traits will be used during mate choice, when preferences for locally adapted traits increase heterospecific mating. In particular, we study the evolution of preference toward an adaptive versus a neutral trait in sympatric species. We take into account sensory trade-offs, which may limit the emergence of preference for several traits. Our model highlights that the evolution of preference toward adaptive versus neutral traits depends on the selective regimes acting on traits but also on heterospecific interactions. When the costs of heterospecific interactions are high, mate preference is likely to target neutral traits that become a reliable cue limiting heterospecific matings. We show that the evolution of preference toward a neutral trait benefits from a positive feedback loop: The more preference targets the neutral trait, the more it becomes a reliable cue for species recognition. We then reveal the key role of sensory trade-offs and the cost of choosiness favoring the evolution of preferences targeting adaptive traits, rather than traits reducing heterospecific mating. When sensory trade-offs and the cost of choosiness are low, we also show that preferences targeting multiple traits evolve, improving offspring fitness by both transmitting adapted alleles and reducing heterospecific mating. Altogether, our model aims at reconciling "good gene" and reinforcement models to provide general predictions on the evolution of mate preferences within natural communities.
Keywords: good gene; mate preference; preference for multiple traits; speciation; species recognition; theory.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Society for the Study of Evolution (SSE) and European Society for Evolutionary Biology (ESEN).
Figures


Similar articles
-
Experimental evidence for asymmetric mate preference and aggression: behavioral interactions in a woodrat (Neotoma) hybrid zone.BMC Evol Biol. 2013 Oct 4;13:220. doi: 10.1186/1471-2148-13-220. BMC Evol Biol. 2013. PMID: 24093823 Free PMC article.
-
The evolution of female mating preferences: differentiation from species with promiscuous males can promote speciation.Evolution. 2006 Oct;60(10):1967-80. Evolution. 2006. PMID: 17133854
-
Evolution of choosiness dictates whether search costs of mate choice enhance speciation by sexual selection.J Evol Biol. 2022 Aug;35(8):1045-1059. doi: 10.1111/jeb.14036. Epub 2022 Jul 13. J Evol Biol. 2022. PMID: 35830473
-
The evolution of male mate choice in insects: a synthesis of ideas and evidence.Biol Rev Camb Philos Soc. 2001 Aug;76(3):305-39. doi: 10.1017/s1464793101005693. Biol Rev Camb Philos Soc. 2001. PMID: 11569787 Review.
-
Variation in mate choice and mating preferences: a review of causes and consequences.Biol Rev Camb Philos Soc. 1997 May;72(2):283-327. doi: 10.1017/s0006323196005014. Biol Rev Camb Philos Soc. 1997. PMID: 9155244 Review.
References
-
- Borzée, A., Kim, J. Y., Da Cunha, M. A. M., Lee, D., Sin, E., Oh, S., Yi, Y., & Jang, Y. (2016). Temporal and spatial differentiation in microhabitat use: Implications for reproductive isolation and ecological niche specification. Integrative Zoology, 11(5), 375–387. doi:10.1111/1749-4877.12200 - DOI - PubMed
-
- Candolin, U., & Reynolds, J. D. (2001). Sexual signaling in the European bitterling: Females learn the truth by direct inspection of the resource. Behavioral Ecology, 12(4), 407–411.

