Effects of duo-strain probiotics on growth, digestion, and gut health in broiler chickens
- PMID: 38525085
- PMCID: PMC10958615
- DOI: 10.1016/j.vas.2024.100343
Effects of duo-strain probiotics on growth, digestion, and gut health in broiler chickens
Abstract
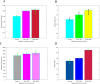
The goal of this inquiry was to analyze the impact of incorporating Enterococcus faecium and Streptococcus thermophilus using a novel premix-spray method on the following aspects: growth rate, digestive enzyme activity, antioxidant levels, gut microbiome composition, and the morphological characteristics of the duodenum, jejunum, and ileum in broiler chickens. Furthermore, this study explored the potential benefits of duo strains of probiotics (DSP) in reducing flatulence, regulating stool microbial population, and improving diarrhea symptoms. A total of 360 one-day-old mixed-sex Plymouth Rock chicks (IW: 51 ± 33 g) were randomly divided into two treatment groups. Each treatment group was further divided into 9 replicated cages, with 20 chicks housed in each cage. The control group (CG) received a basal diet composed of a soy-corn mixture, whereas the experimental group was provided with DSP (CON + 0.5 % probiotic). The results showed that the increase in the body weight of broilers at the end of the fourth week in the control group and the treatment group was 1.576 versus 1.847 kg, respectively. Throughout the 30-day trial period, the DSP diet significantly improved the specific growth rate (SGR), survival rate (SR), and body weight gain (BWG) while decreasing the feed conversion ratio (FCR) (P < 0.05). The DSP diet also enhanced the Enzymatic digestion (protease, amylase, lipase, and trypsin) and antioxidant potential (SOD, MDA, and catalase) of the broilers compared to those in the CG. The results revealed significant enhancements in the tissue morphology of the duodenum and jejunum following the combined treatment for a duration of 4 weeks. The DSP treatments significantly increased microvillus height in the duodenum and jejunum but had no notable effects in the ileum. Incorporating 0.5 % DSP in poultry feed improved the relative abundance of Ruminococcaceae and Faecalibacteriumin, leading to better management of diarrhea and reduced presence of E. coli compared to the control diet. Additionally, including probiotics in the basal diet reduced H2S, CO2, NH3, and CH4 levels. Overall, the study suggests that the new spray-drying approach with these strains has potential for supplementing probiotics in poultry feed processing, and including DSP in broiler chicken diets has beneficial effects.
Keywords: Antioxidant levels; Broiler; Duo-strain probiotics; Enzymatic digestion; Growth rate; Gut Microbiome.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Archacka M., Celińska E., Białas W. Techno-economic analysis for probiotics preparation production using optimized corn flour medium and spray-drying protective blends. Food and Bioproducts Processing. 2020;123:354–366. doi: 10.1016/j.fbp.2020.07.002. - DOI
-
- Carron M., Alarcon P., Karani M., Muinde P., Akoko J., Onono J., Fèvre E.M., Häsler B., Rushton J. The broiler meat system in Nairobi, Kenya: Using a value chain framework to understand animal and product flows, governance and sanitary risks. Preventive Veterinary Medicine. 2017;147:90–99. doi: 10.1016/j.prevetmed.2017.08.013. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

