The effect of marker types and density on genomic prediction and GWAS of key performance traits in tetraploid potato
- PMID: 38525152
- PMCID: PMC10957621
- DOI: 10.3389/fpls.2024.1340189
The effect of marker types and density on genomic prediction and GWAS of key performance traits in tetraploid potato
Abstract
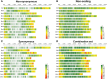
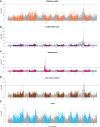
Genomic prediction and genome-wide association studies are becoming widely employed in potato key performance trait QTL identifications and to support potato breeding using genomic selection. Elite cultivars are tetraploid and highly heterozygous but also share many common ancestors and generation-spanning inbreeding events, resulting from the clonal propagation of potatoes through seed potatoes. Consequentially, many SNP markers are not in a 1:1 relationship with a single allele variant but shared over several alleles that might exert varying effects on a given trait. The impact of such redundant "diluted" predictors on the statistical models underpinning genome-wide association studies (GWAS) and genomic prediction has scarcely been evaluated despite the potential impact on model accuracy and performance. We evaluated the impact of marker location, marker type, and marker density on the genomic prediction and GWAS of five key performance traits in tetraploid potato (chipping quality, dry matter content, length/width ratio, senescence, and yield). A 762-offspring panel of a diallel cross of 18 elite cultivars was genotyped by sequencing, and markers were annotated according to a reference genome. Genomic prediction models (GBLUP) were trained on four marker subsets [non-synonymous (29,553 SNPs), synonymous (31,229), non-coding (32,388), and a combination], and robustness to marker reduction was investigated. Single-marker regression GWAS was performed for each trait and marker subset. The best cross-validated prediction correlation coefficients of 0.54, 0.75, 0.49, 0.35, and 0.28 were obtained for chipping quality, dry matter content, length/width ratio, senescence, and yield, respectively. The trait prediction abilities were similar across all marker types, with only non-synonymous variants improving yield predictive ability by 16%. Marker reduction response did not depend on marker type but rather on trait. Traits with high predictive abilities, e.g., dry matter content, reached a plateau using fewer markers than traits with intermediate-low correlations, such as yield. The predictions were unbiased across all traits, marker types, and all marker densities >100 SNPs. Our results suggest that using non-synonymous variants does not enhance the performance of genomic prediction of most traits. The major known QTLs were identified by GWAS and were reproducible across exonic and whole-genome variant sets for dry matter content, length/width ratio, and senescence. In contrast, minor QTL detection was marker type dependent.
Keywords: GBLUP; GWAS; Solanum tuberosum; genomic prediction; marker density; marker type; tetraploid potato breeding.
Copyright © 2024 Aalborg, Sverrisdóttir, Kristensen and Nielsen.
Conflict of interest statement
Author HK is currently employed by Arla Foods Denmark. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bates D., Mächler M., Bolker B. M., Walker S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67. doi: 10.18637/jss.v067.i01 - DOI
LinkOut - more resources
Full Text Sources

